Difference: Fluid and Slime
Liquids consist of many small particles that are densely packed together but can move freely against each other. In thin liquids, the particles are usually small and move past each other quite unhindered. In viscous liquids, the particles are larger and often contain long chains that intertwine.
A good slime is wobbly, can be kneaded, formed, torn, and adheres easily to surfaces such as fingers without leaving any residue. It is a gel. A gel consists of at least two components: One component is solid and forms a 3D network. The other component is a liquid or gas that fills the pores of the network.
Original Slime
The original slime or DIY slime contains polyvinyl alcohol (PVA), borax (sodium tetraborate), and water. If borax is dissolved in water, boric acid is formed, which reacts further to tetrahydroxyborate. Tetrahydroxyborate reacts with PVA. The OH groups of both particles combine to form solid compounds so that the borate anions become “nodes” in a flexible network. In addition, water is formed which is stored in the pores of the network.
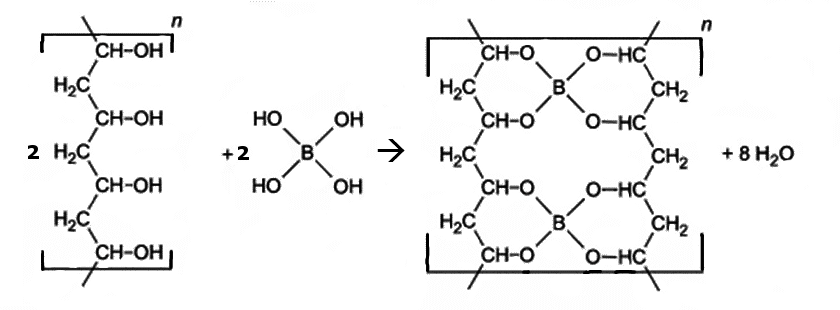
The bonds in the PVA borate network can be broken and newly formed relatively easily. Thus, the network is very changeable during kneading. This makes the slime really slimy.
Disposal
Borax and other boric acid compounds can damage unborn babies in the womb and impair fertility. Therefore, they are no longer sold to private households in EU countries and are banned from school laboratories. Borax must be disposed of as hazardous waste. If slime residues contain borax, they should be disposed of as hazardous waste.
Baking soda may be used as a substitute for borax.
Also of Interest
- Chemistry Makes Slime Glow in the Dark,
ChemistryViews.org 2018.




