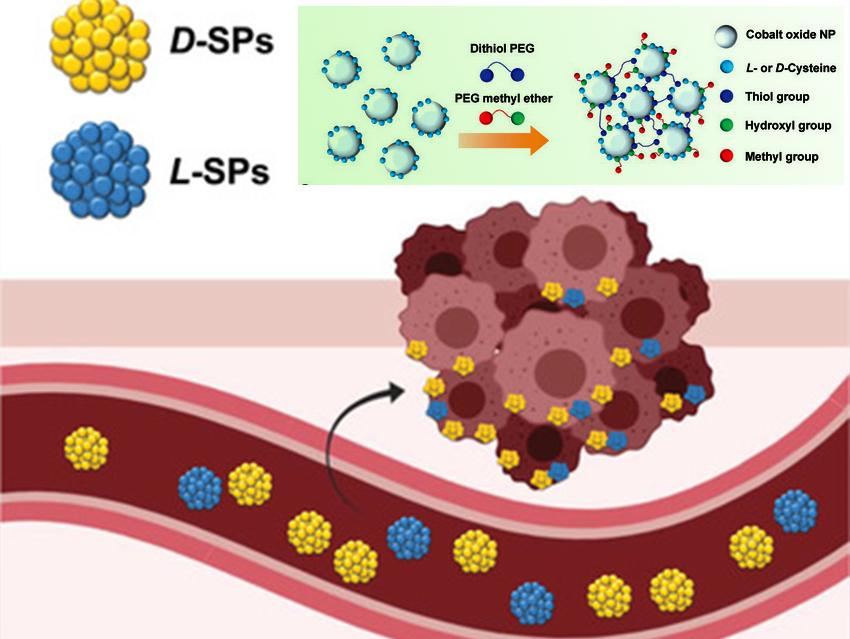
Controlling biomaterial chirality to influence interactions with cells is of great interest. In contrast to molecular chirality, supramolecular chirality and its potential role in biology remain poorly understood.
Robert Langer and Ana Jaklenec, David H. Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology (MIT), Cambridge, MA, USA, and colleagues have shown that incorporating d‐chirality into nanosystems improves cellular uptake and in vivo stability in blood. The team synthesized chiral supraparticles (SPs) through self‐limiting assembly. For this, they manipulated the attractive and repulsive interactions between nanoparticles (NPs) building blocks (pictures top right).
l‐ and d‐cysteines were used as chirality providing agents and cobalt oxide as NPs building blocks. Dithiol poly(ethylene glycol) (PEG) and PEG methyl ether were added to drive attractive and repulsive competing interactions between the NPs. These interactions can lead to both self‐assembly and self‐terminating SPs. Due to the strong affinity of thiol groups to cobalt, dithiol PEG works as a cross‐linker. Hydroxyl groups bind on the NPs’ surface when the methyl group is exposed and then provide a slight repulsive force. When the attractive and repulsive interactions are balanced, the growth of SPs stops. Growth and self‐assembly occurred spontaneously in the aqueous phase at room temperature for 2 h. SPs achieved a size of ≈60 nm when equimolar amounts of dithiol PEG and PEG methyl ether were used.
Due to the stronger affinity between the same optical isomeric systems, SPs capped by d‐cysteine showed a higher attractive and adhesive interaction with cellular membranes that are composed mainly of lipid molecules. This leads to a 3–4‐fold increase in cell membrane penetration in breast, cervical, and multiple myeloma cancer cells. SPs capped by d‐cysteine showed superior stability and longer biological half‐lives in blood. This is likely due to the opposite chirality and thus protection from endogenous proteins including proteases.
According to the researchers, chiral nanosystems may have the potential to provide a new level of control for drug delivery systems (DDS), tumor detection markers, biosensors, and potentially other biomaterial devices.
- Chiral Supraparticles for Controllable Nanomedicine,
Jihyeon Yeom, Pedro P. G. Guimaraes, Hyo Min Ahn, Bo‐Kyeong Jung, Quanyin Hu, Kevin McHugh, Michael J. Mitchell, Chae‐Ok Yun, Robert Langer, Ana Jaklenec,
Adv. Mater. 2019.
https://doi.org/10.1002/adma.201903878