In living beings, metalloenzymes catalyze oxidation reactions using atmospheric oxygen. As an abundant and environmentally friendly oxidant, O2 is also an attractive reagent for organic synthesis. However, due to its radical ground state, which leads to radical reaction pathways, the use of O2 for selective reactions is often difficult. One possible strategy to overcome these difficulties is the use of molecular oxygen coordinated to transition metal centers, which suppresses undesirable reactions.
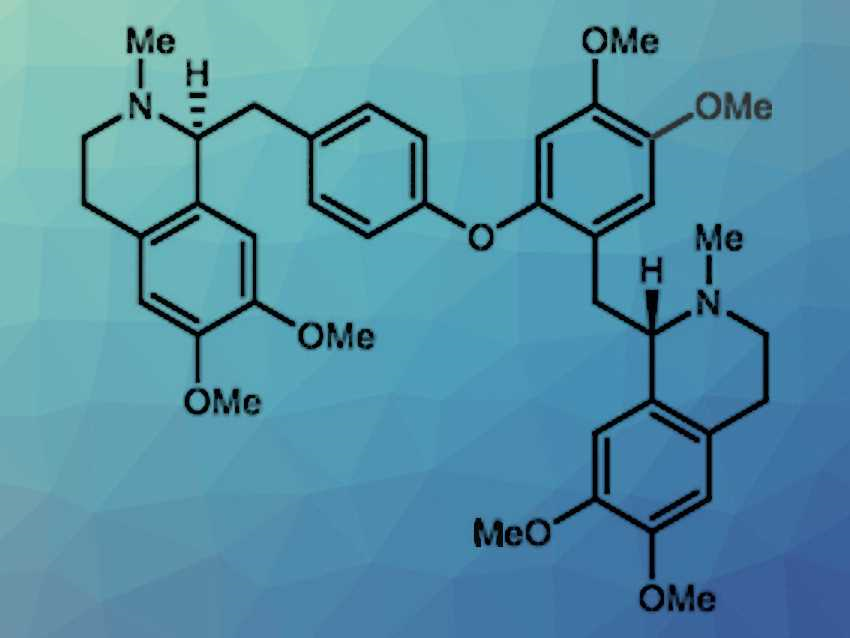
Jean Phillip Lumb, McGill University, Montreal, Canada, and colleagues have used this strategy to develop a total synthesis for the alkaloid (S,S)-tetramethylmagnolamine (pictured). Inspired by the copper-based enzyme tyrosinase, the researchers developed a catalyst system using the commercially available reagents tetrakis(acetonitrile)copper(I)hexafluorophosphate, (Cu(CH3CN)4)PF6, and N,N‘-di-tert-butylethylenediamine (DBED). This catalytic system can oxidize para-substituted phenols in the ortho position. The system gives a semiquinone radical complex, which goes on to form an oxygen-bridged species when another equivalent of phenol is added.
The researchers used this reaction to synthesize (S,S)-tetramethylmagnolamine. Specifically, they used it to build the central ether bridge of the target molecule from two equivalents of a phenolic building block in dichloromethane under 1 atmosphere of oxygen at room temperature. After reduction and deprotection, the target product was obtained in a total yield of 21 %. With this, the team showed that the catalytic oxidation system can be successfully used in aerobic desymmetrization reactions and can, therefore, be a useful tool in the total synthesis of natural products.
- Total Synthesis of (S,S)-Tetramethylmagnolamine via Aerobic Desymmetrization,
Zheng Huang, Xiang Ji, Jean-Philip Lumb,
Org. Lett. 2019.
https://doi.org/10.1021/acs.orglett.9b03559