The efficient sunlight-driven production of hydrogen from water would be an important step towards sustainable energy generation. Such processes require the coupling of water oxidation and proton reduction, which still is challenging.
The ideal hydrogen evolution catalyst should be made from abundant materials, be easy to synthesize, be of low toxicity, and work in water, preferably at neutral pH. The latter is a challenge for many metal complexes because they are either not water-soluble or subject to degradation under aqueous conditions. Cobalt-based catalytic systems have already shown promising results in water, and some of these systems work close to neutral pH.
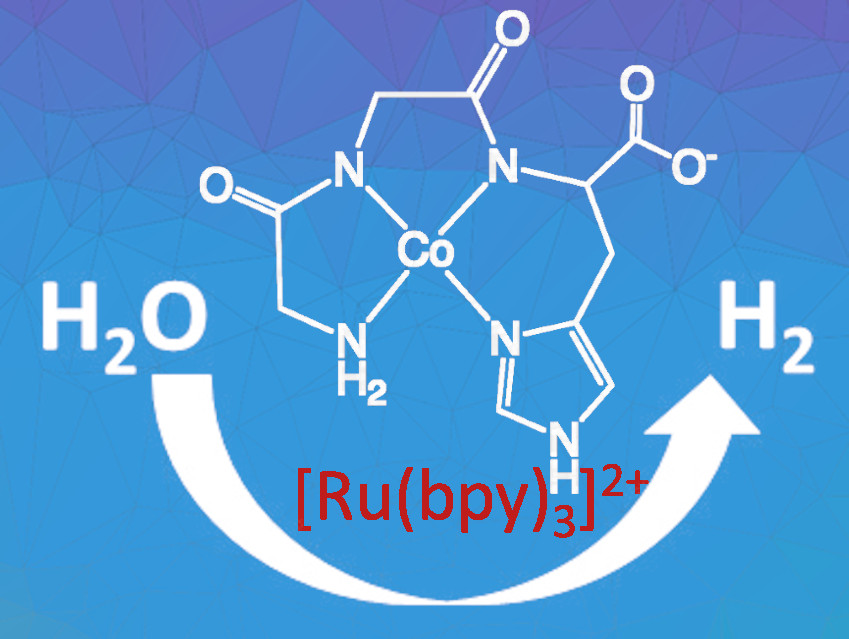
Kara L. Bren, University of Rochester, NY, USA, and colleagues have developed a photochemical hydrogen evolution system using the cobalt complex of the tripeptide Gly-Gly-His (pictured). This peptide fragment coordinates the cobalt center through the N-terminal amine, the amide nitrogen atoms, and the histidine’s imidazole moiety. The researchers combined this complex with the commonly used photosensitizer tris(bipyridine)ruthenium(II) chloride, [Ru(bpy)3]Cl2. Ascorbate was used as a sacrificial reductant. The addition of sacrificial oxidants or reductants allows the separate investigation of the water oxidation and proton reduction reactions.
Hydrogen evolution studies were carried out in water buffered by 3-(N-morpholino)propanesulfonic acid (MOPS) at 15 °C under an oxygen-free atmosphere. The system was irradiated with either blue or green light. The catalyst is active under both conditions: blue light gives higher turnover numbers, while longevity is improved under green light. The catalytic system can generate hydrogen at near-neutral pH with a turnover number of up to 2,200 for over 40 hours. The addition of fresh photosensitizer showed that the loss of activity after this time is due to the degradation of the photosensitizer, while the catalyst itself remains stable.
- Photochemical Hydrogen Evolution from Neutral Water with a Cobalt Metallopeptide Catalyst,
Saikat Chakraborty, Emily H. Edwards, Banu Kandemir, Kara L. Bren,
Inorg. Chem. 2019.
https://doi.org/10.1021/acs.inorgchem.9b02067