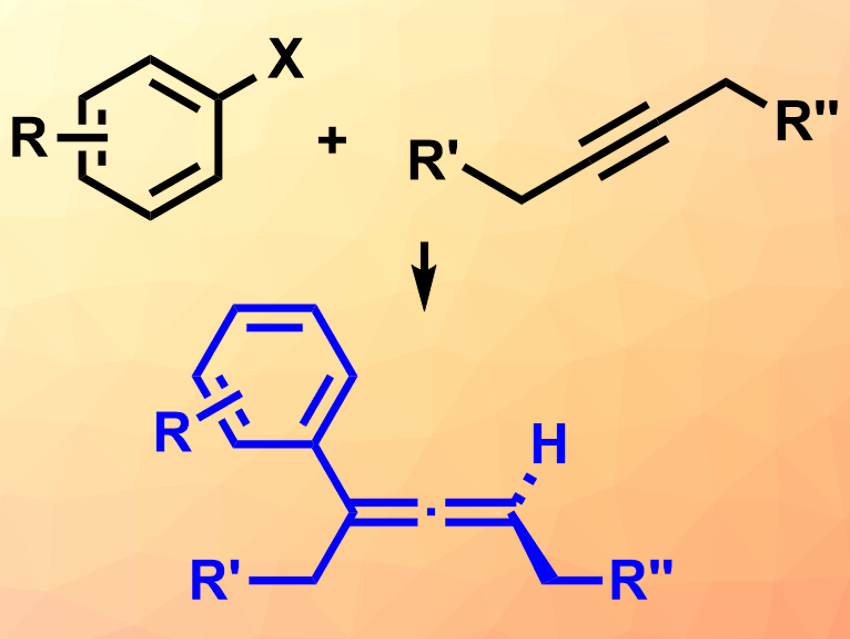
Allenes, i.e., organic molecules with two neighboring C=C bonds, can be chiral. They are useful synthetic intermediates and can be found in natural products. There are several strategies for the synthesis of chiral trisubstituted allenes. However, it is still challenging to use the well-known Heck reaction to create allenes in an enantioselective way. This is due to an unfavored β-hydride elimination at an intermediate vinyl palladium species.
Shengming Ma, Fudan University, Shanghai, China, Junliang Zhang, East China Normal University, Shanghai, and Fudan University, and colleagues have developed the first palladium-catalyzed asymmetric Heck reaction between aryl triflates and alkynes to give trisubstituted allenes (pictured). The team used di-μ-chloro-bis[2-[(dimethylamino)methyl]phenyl-C,N]dipalladium(II) as a catalyst, together with a chiral sulfinamide phosphine ligand with large aromatic substituents (a modified Xu-Phos ligand). The reaction was performed in the presence of Na3PO4 as a base in a mixture of tetrahydrofuran (THF) and water at 65 °C.
According to the team, the steric hindrance induced by the large substituents at the sulfinamide phosphine ligand allows the otherwise energetically unfavored β-hydride elimination. The desired products were obtained in moderate to high yields and with high enantioselectivities. These products could be useful in further transformations to create other chiral compounds.
- Pd-Catalyzed Enantioselective Heck Reaction of Aryl Triflates and Alkynes,
Chenghao Zhu, Haoke Chu, Gen Li, Shengming Ma, Junliang Zhang,
J. Am. Chem. Soc. 2019.
https://doi.org/10.1021/jacs.9b10883