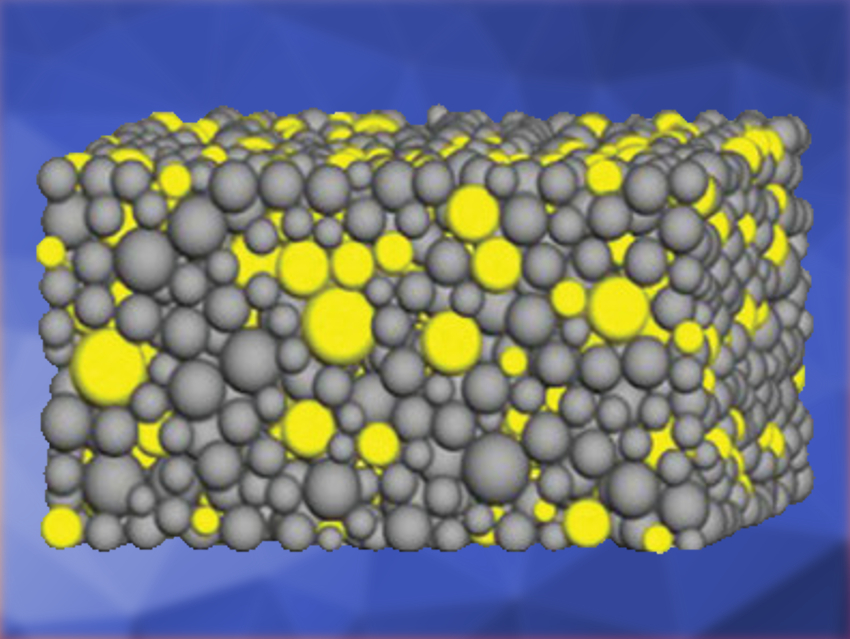
Solid-state batteries are a promising alternative to conventional lithium-ion batteries. Instead of a flammable organic electrolyte, a solid lithium-ion conductor is used. The solid composite cathode in these batteries is made from a mixture of the cathode active material (CAM), the solid electrolyte (SE), and carbon. The volume fraction of the CAM in the mixture is called the cathode loading. The energy density of the cell increases with higher cathode loading. Usually, a high volume fraction of SE must be used for efficient ionic diffusion, resulting in a lower cathode loading and a lower energy density.
Gerbrand Ceder, University of California and Lawrence Berkeley National Laboratory, Berkeley, USA, and colleagues have found a way to increase the cathode loading of cold-pressed solid-state batteries via particle size optimization. The researchers used Li2O–Zr2O (LZO)-coated LiNi0.5Mn0.3Co0.2O2 (NMC) as the CAM and glassy 75Li2S–25P2S5 (LPS) as the SE. The particle sizes of NMC and LPS were varied to find the optimal ratio. For this, the team used both modeling and experiments.
The team modeled the cathode microstructure (pictured) by the random insertion of differently sized particles into a cubic simulation domain. Then, the corresponding diffusion pathways of lithium ions and the cathode utilization were calculated. The model calculations suggest that the cathode utilization and, therefore, the energy density, depends on the particle-size ratio of CAM to SE. At a fixed cathode loading, cathode utilization increases with a higher particle-size ratio. This means that the particle size of the CAM must be bigger than that of the SE. The calculations were confirmed by the experimental results.
Based on these results, it is possible to increase the capacity of a solid-state battery by merely changing particle sizes. The researchers fabricated a solid-state cathode composite with a cathode loading over 50 vol%, comparable to the cathode of a liquid-electrolyte battery.
- High Active Material Loading in All-Solid-State Battery Electrode via Particle Size Optimization,
Tan Shi, Qingsong Tu, Yaosen Tian, Yihan Xiao, Lincoln J. Miara, Olga Kononova, Gerbrand Ceder,
Adv. Energy Mater. 2019.
https://doi.org/10.1002/aenm.201902881