Lithium–sulfur (Li–S) batteries are not as widely used as lithium-ion batteries, but they have a much higher theoretical energy density. The energy generation mechanism in Li–S batteries creates polysulfide ions that shuttle between the electrodes and react with the Li anode—the aptly named “polysulfide shuttle”. This reduces the long-term efficiency of the batteries. This problem needs to be solved before Li–S batteries can be used on a large commercial scale.
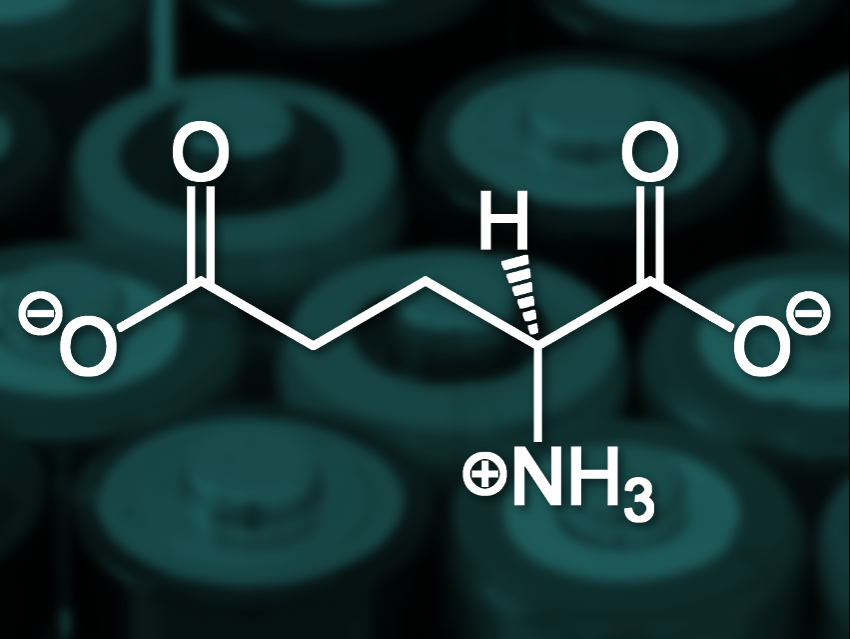
Weidong He, Chunhui Yang, Harbin Institute of Technology, China, and colleagues have developed a glutamate electrolyte to be used in Li–S batteries. The team dissolved glutamate (pictured) in commonly used electrolytes, such as the lithium salt of bis(trifluoromethanesulfonyl) imide (LiTFSI). The negatively charged glutamate has a repulsive effect on polysulfides, which suppresses the shuttle effect.
The glutamate-containing electrolyte prevents the rapid capacity decay, self-discharge, and poor high-temperature performance that are usually caused by the shuttling of polysulfide ions. Li–S batteries with the improved electrolyte showed a capacity retention of 60 % after 1,000 cycles, a much lower self-discharge rate than that of conventional batteries, and stable operation at 60 °C.
- Suppression of Polysulfide Dissolution and Shuttling with Glutamate Electrolyte for Lithium Sulfur Batteries,
Liwei Dong, Jipeng Liu, Dongjiang Chen, Yupei Han, Yifang Liang, Mengqiu Yang, Chunhui Yang, Weidong He,
ACS Nano 2019.
https://doi.org/10.1021/acsnano.9b06934