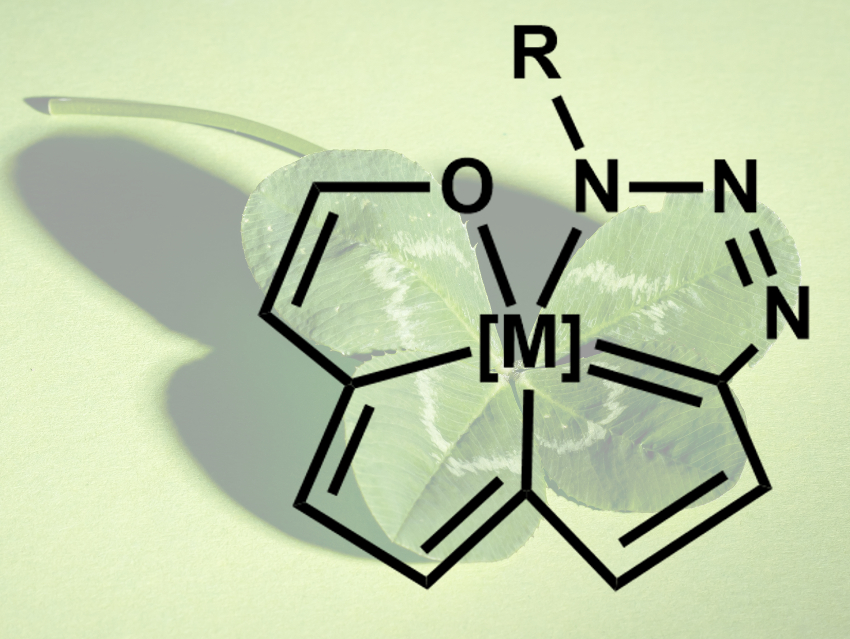
Aromatic molecules with more than three fused rings sharing a single “bridgehead” atom are challenging to create. This is due to a limitation of the central carbon atom, which can only be involved in up to three aromatic rings at once. Other elements, however, could, be used to create tetracyclic aromatic compounds.
Haiping Xia, Xiamen University and Southern University of Science and Technology, Shenzhen, both China, Hong Zhang, Xiamen University, and colleagues have synthesized a new class of tetracyclic aromatics with a metal atom at the center (general structure pictured). The team started from tricyclic osmium chloro complexes, which were converted to carbyne complexes of the type [M]≡C–R. A 1,3-dipolar cycloaddition with an azide then gave the desired tetracyclic compounds in yields up to 92 %, depending on the substituent at the azide.
The products are stable both in solution and in the solid state. The osmium center is coordinated in a pentagonal bipyramidal structure and serves as a common “bridgehead” for all four five-membered rings. The team performed density functional theory (DFT) calculations on model systems and found evidence for aromatic properties. According to the researchers, this would make the complexes the first examples of a transition metal center shared by four aromatic units simultaneously.
- Access to tetracyclic aromatics with bridgehead metals via metalla-click reactions,
Zhengyu Lu, Qin Zhu, Yuanting Cai, Zhixin Chen, Kaiyue Zhuo, Jun Zhu, Hong Zhang, Haiping Xia,
Sci. Adv. 2020, 6, eaay2535.
https://doi.org/10.1126/sciadv.aay2535