The loss of lithium from the cathode during the first charging cycles is a problem in lithium-ion batteries. During these first cycles, lithium from the cathode material is irreversibly bound at the surface of the anode. This process reduces the overall cell capacity and limits the performance of the battery. Additives functioning as so-called sacrificial cathode materials can provide extra lithium ions to the cathode by reacting during the first charge, compensating for lost lithium ions. However, many of those additives develop gases, which could be dangerous.
Maria Diaz-Lopez, Rutherford Appleton Laboratory and Diamond Light Source Ltd., Didcot, UK, and colleagues have developed a sacrificial cathode additive made from a nanoscale mixture of Li2O and Li2/3Mn1/3O5/6, which shows a disordered and non-stoichiometric MnO-type rock-salt (RS) structure. The team prepared two composites with a Li2O content of 35 mol% and 55 mol%, respectively, using a mechanochemical approach. The composites have very high first-charge capacities of 898 and 1157 mAh g–1, respectively.
The team monitored the sacrificial cathode reaction by in situ total scattering. During the first charging cycles, they observed a contraction and expansion of the RS lattice mimicking the electrochemical curve. This can be explained with the release and incorporation of lithium ions. The signals of Li2O disappear permanently after the first charge, which means it irreversibly reacts. According to the researchers, the exceptionally high first-charge capacities are caused by this reaction. No gas evolution or secondary phases were noted.
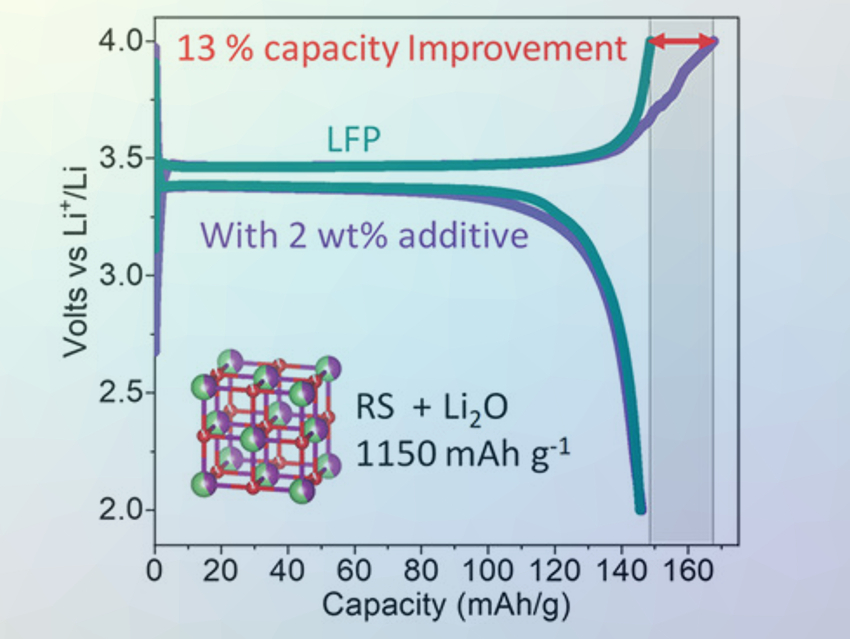
The team tested the most effective composite with 55 mol% Li2O as a sacrificial cathode material. It was added in a concentration of 2 wt% to the common cathode materials LiFePO4 and LiCoO2, which were cycled against lithium metal. The first charge capacity was improved by 13 % without decreasing the discharge capacity. This work could lead to a new class of highly effective sacrificial additives without dangerous gas release.
- Li2O:Li–Mn–O Disordered Rock-Salt Nanocomposites as Cathode Prelithiation Additives for High-Energy Density Li-on Batteries,
Maria Diaz-Lopez, Philip A. Chater, Pierre Bordet, Melanie Freire, Christian Jordy, Oleg I. Lebedev, Valerie Pralong,
Adv. Energy Mater. 2020.
https://doi.org/10.1002/aenm.201902788