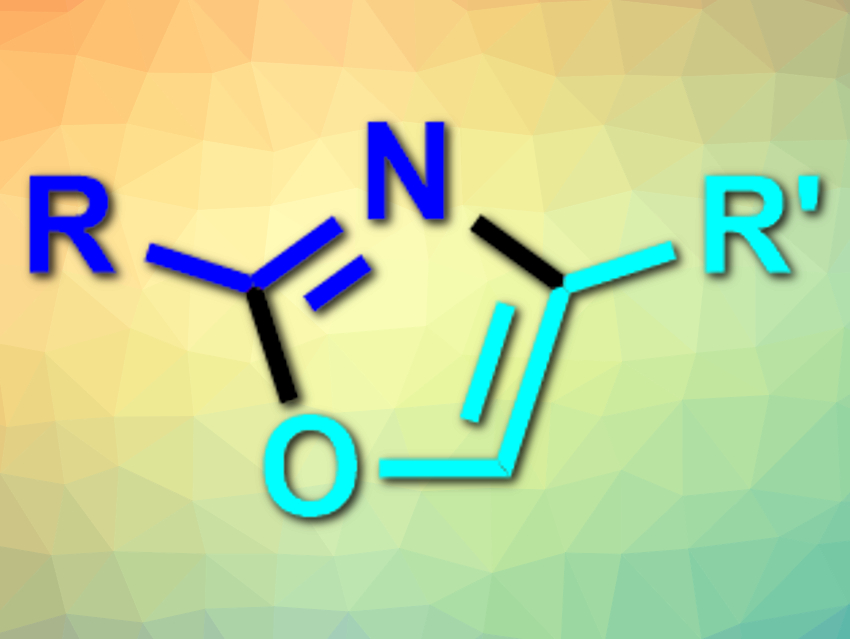
Five-membered heterocycles such as oxazoles (pictured), imidazoles, and thiazoles are often found in pharmaceutically active compounds. They are usually synthesized from, e.g., amines and carbonyl derivatives via condensation reactions.
David A. Nagib, and colleagues, The Ohio State University, Columbus, USA, have developed an alternative approach to the synthesis of azoles. The team used simple chemical feedstocks, i.e., alcohols and amines, as starting materials and used a radical cascade reaction to form the desired oxazoles. The reaction involves a double hydrogen-atom transfer (HAT). The team first prepared imidates from the reactants using triflic acid. The imidates were then converted to the desired oxazoles via a radical process using CsI and PhI(OAc)2 in toluene under irradiation with light.
The products were obtained in good to excellent yields and the reaction can be performed in a one-pot process. Other azoles such as imidazoles could also be prepared using this approach. According to the researchers, the reaction proceeds via the formation of an imidate radical, which undergoes a C–H amination via a hydrogen-atom transfer. The resulting cyclized product undergoes a second hydrogen-atom transfer and an aromatization to give the oxazole product.
- Radical cascade synthesis of azoles via tandem hydrogen atom transfer,
Andrew D. Chen, James H. Herbort, Ethan A. Wappes, Kohki M. Nakafuku, Darsheed N. Mustafa, David A. Nagib,
Chem. Sci. 2020.
https://doi.org/10.1039/C9SC06239D