Möbius aromaticity is a special type of aromaticity that usually exists in twisted organic molecules that resemble a Möbius strip. The rules for the number of electrons in their π-system are the opposite of “normal” Hückel aromatics: Systems with 4n π-electrons are aromatic, while those with 4n+2 π-electrons are anti-aromatic (with n being a positive integer).
The twist in the molecules causes a phase change in its p-orbitals, which allows for the Möbius aromaticity. Thus, there usually are no planar Möbius-aromatic systems. However, if the molecule contains a transition-metal atom, the phase change can be induced by a d-orbital without any twist in the structure. This makes Möbius aromaticity possible in a planar ring.
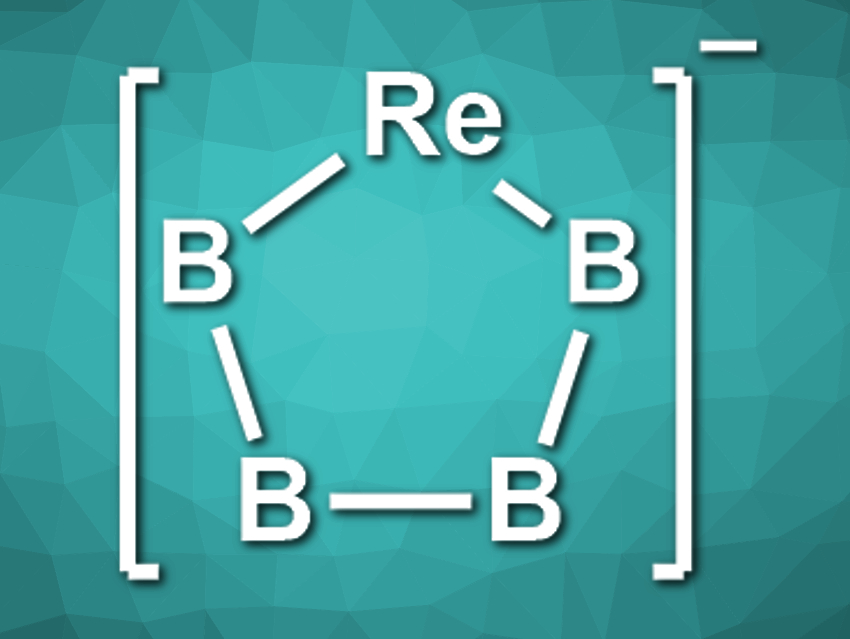
Lai-Sheng Wang, Brown University, Providence, RI, USA, and colleagues have observed the first planar monocyclic metallaboron system with Möbius aromaticity, ReB4– (pictured). The team ablated a target made of Re, B, and Ag as a binder with a laser to synthesize the desired compound. They used a high-resolution photoelectron spectroscopy setup to characterize the products.
The researchers found two rhenium-boride clusters: ReB3–, which has a pyramidal structure, and ReB4–, which has a planar pentagonal structure with four π-electrons. Calculations of the chemical shifts confirm the Möbius-aromatic properties of ReB4–. According to the team, the results indicate that there could be a new class of Möbius-aromatic metallaborons with other metals.
- Observation of Möbius Aromatic Planar Metallaborocycles,
Ling Fung Cheung, G. Stephen Kocheril, Joseph Czekner, Lai-Sheng Wang,
J. Am. Chem. Soc. 2020.
https://doi.org/10.1021/jacs.9b13417