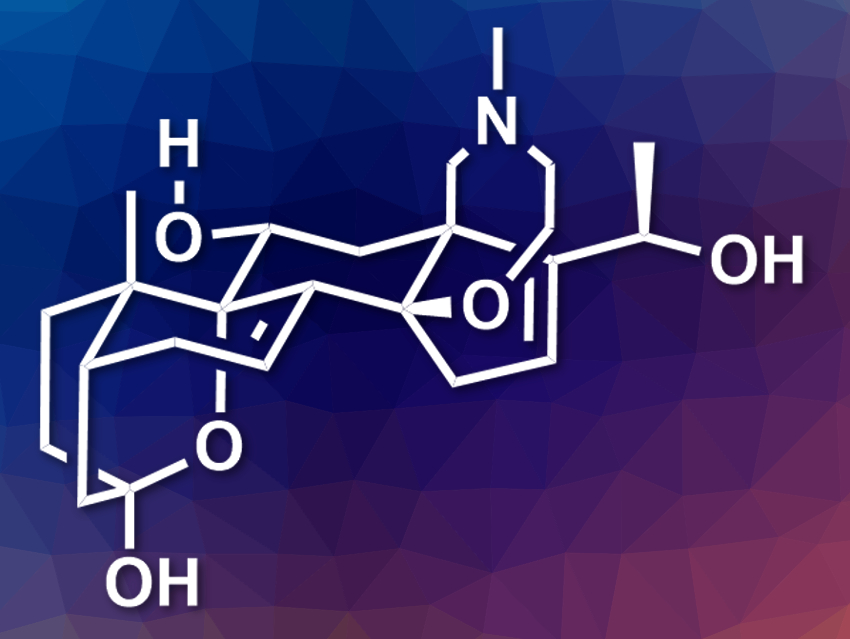
(−)-Batrachotoxin is a highly toxic alkaloid first found on poison-dart frogs in South America. It is an important tool for studying ion transport across cell membranes, specifically voltage-gated sodium channels. (−)-Batrachotoxinin A (pictured) is a less potent derivative. It could be converted to analogues that are also useful for the study of sodium transport. However, the total synthesis of these compounds is challenging due to their complex structure and highly oxidized nature.
Tuoping Luo, Peking University, Beijing, China, and colleagues have performed an efficient enantioselective total synthesis of (−)-batrachotoxinin A. The team started from ethyl vinyl ether, a bicyclic diketone, a bicyclic α-bromoketone, methylamine, and chloroacetyl chloride. The bicyclic systems were coupled using a photoredox coupling reaction and the resulting intermediate was subjected to a local-desymmetrization operation to create the steroid skeleton.
The researchers were able to perform the synthesis on a gram scale until a late-stage intermediate. Due to the toxicity of the product, they chose a smaller-scale reaction for the final steps. According to the team, the synthesis could also be used to prepare other highly oxidized natural products.
- Total Synthesis of (−)-Batrachotoxinin A: A Local-Desymmetrization Approach,
Yinliang Guo, Zhixian Guo, Jia-Tian Lu, Runting Fang, Si-Cong Chen, Tuoping Luo,
J. Am. Chem. Soc. 2020.
https://doi.org/10.1021/jacs.9b12882