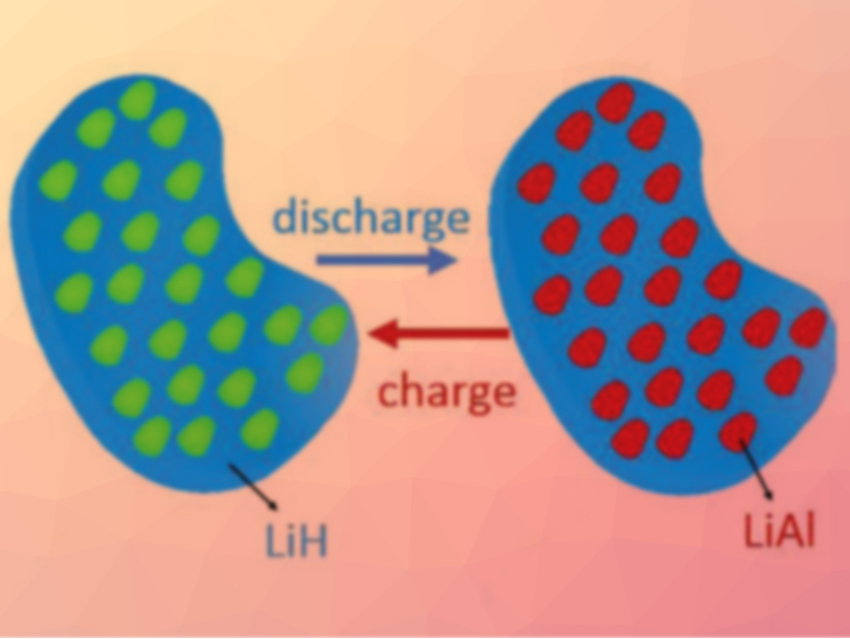
Lithium aluminum hydride (LiAlH4) and related compounds could make good anode materials for lithium-ion-batteries because of their low weight and high specific capacity. However, LiAlH4 cannot be regenerated by an electrochemical reaction because it is thermodynamically unstable. The lithiation of Li3AlH6 is theoretically reversible, but its reactions are too slow for an application in lithium-ion batteries.
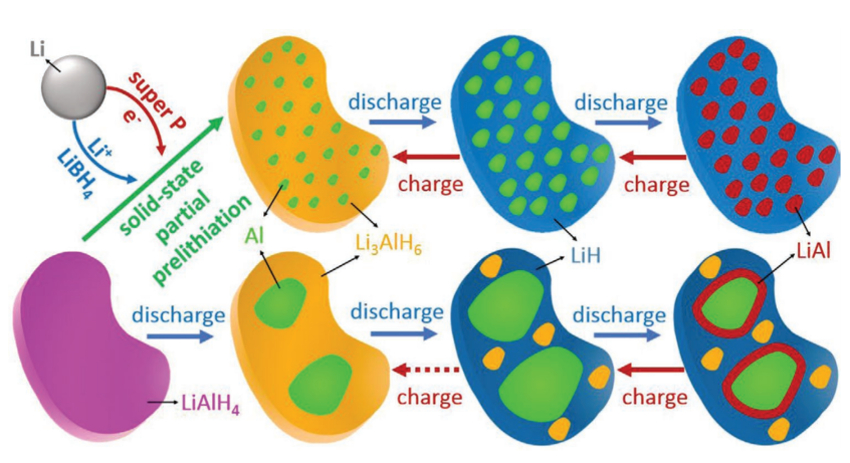
Shiyou Zheng, University of Shanghai for Science and Technology, China, and colleagues have developed a strategy to make this type of electrode material usable by solid-state prelithiation and nanosizing. The researchers mixed lithium metal and LiAlH4 with the ionic conductor LiBH4 and carbon as an electronic conductor. Then, they heated the mixture to 120 °C. Reference electrodes with crystalline Li3AlH6 were made from lithium hydride and LiAlH4 by ball-milling.
During the prelithiation, a composite of aluminum nanograins dispersed in an amorphous matrix was formed (pictured below in green and yellow, respectively). The team described the reaction as a short-circuited electrochemical reaction between Li and LiAlH4. Usually, those compounds do not react in the solid state, but the researchers achieved the reaction by addition of the electronic and ionic conductors. Infrared (IR) spectroscopy, NMR spectroscopy and high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) suggest that the amorphous phase is Li3AlH6.
In contrast to the reference anode, the new anode material can be fully and reversibly lithiated with an initial specific discharge capacity of 2266 mAh g–1. A full cell with a LiCoO2 cathode showed promising properties, with an initial Coulombic efficiency of 88 %, a high cycling stability, and a good rate capability.
- Solid-State Prelithiation Enables High-Performance Li-Al-H Anode for Solid-State Batteries,
Yuepeng Pang, Xitong Wang, Xinxin Shi, Fen Xu, Lixian Sun, Junhe Yang, Shiyou Zheng,
Adv. Energy Mater. 2020.
https://doi.org/10.1002/aenm.201902795