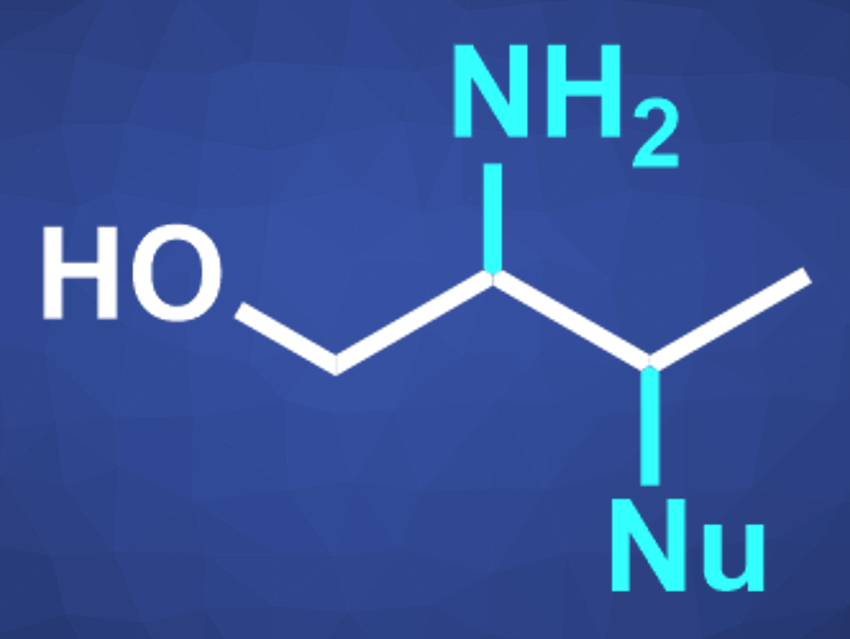
Organic compounds with multiple different functional groups can be useful, e.g., in pharmaceutical chemistry or as intermediates for further reactions. β-Amino alcohols, for example, combine –OH and –NR2 groups and are usually prepared from alkenes.
David A. Nagib, The Ohio State University, Columbus, USA, and colleagues have developed a method for the synthesis of β,γ-functionalized alcohols (pictured) via double C–H functionalization. They converted a range of aliphatic alcohols to a variety of β,γ-substituted alcohols, which bear heteroatoms on three adjacent carbons. The reaction proceeds via a radical-polar-crossover cascade.
First, an alcohol is converted to an N-iodo-imidate by coupling it with a nitrile and reacting it with AcOI, which is prepared in situ from sodium iodide and PhI(OAc)2. The N-iodo-imidate can then react further, initiated by visible light that splits the N–I bond. The resulting N-based radical initiates a 1,5-hydrogen atom transfer (HAT), followed by the formation of a β-iodinated intermediate. This intermediate is converted to an allyl intermediate. Finally, this intermediate undergoes a cyclization to give a γ-iodo oxazoline, which can be hydrolyzed to give the desired substituted alcohol.
The reaction can be performed as a one-pot process. The γ-iodo substituent in the products can be replaced by a variety of nucleophiles, further broadening the range of possible products.
- Vicinal, Double C–H Functionalization of Alcohols via an Imidate Radical-Polar Crossover Cascade,
Allen F. Prusinowski, Raymond K. Twumasi, Ethan A. Wappes, David A. Nagib,
J. Am. Chem. Soc. 2020.
https://doi.org/10.1021/jacs.0c01318