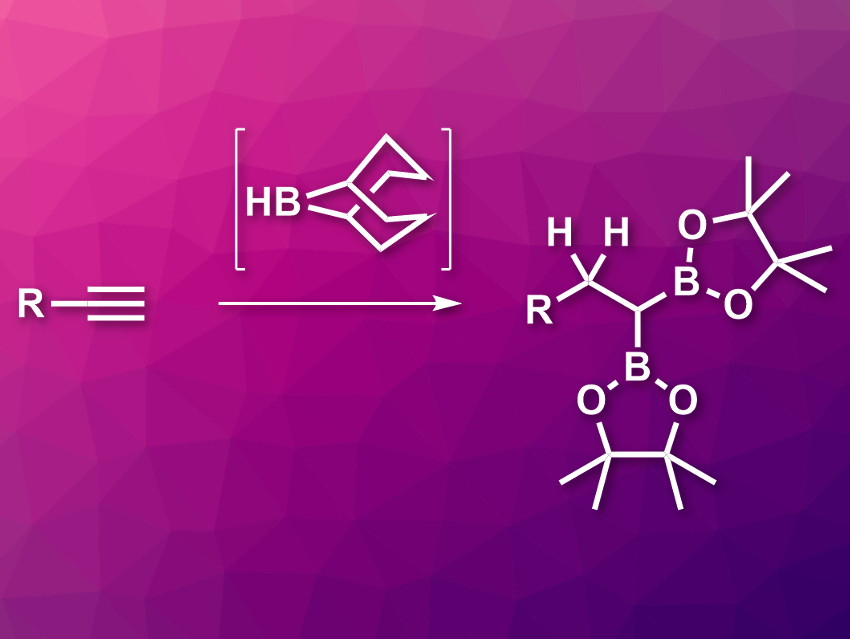
Alkenes and alkynes can undergo hydroboration reactions with many organoborane compounds of the type H–BR2. However, compounds of the type H–B(OR)2, such as pinacolborane (HBpin), do not react spontaneously with C–C multiple bonds. This reaction requires a catalyst, such as a transition metal or another borane. When boranes are used as catalysts, the limiting reaction step is usually the transborylation, i.e., the exchange of one boron group for another at an sp2-hybridized carbon atom. So far, there had been no examples of this type of reaction that involve a transborylation at an sp3-hybridized carbon atom.
Stephen P. Thomas, University of Edinburgh, UK, and colleagues have developed a double hydroboration of alkynes using pinacolborane (HBpin, reaction pictured above). The reaction is catalyzed by the commonly used organoborane reagent 9-borabicyclo[3.3.1]nonane (9-BBN, pictured center) and proceeds via a transborylation at an sp3-hybridized carbon atom. The team used a wide variety of terminal alkynes as substrates and reacted them with HBpin in the presence of 9-BBN without an additional solvent at 120 °C.
The desired gem-diboryl alkanes were obtained in moderate to excellent yields. According to the team, the mechanism involves a transborylation from a secondary alkyl–9-BBN intermediate. This step needs a lot of energy, which is responsible for the required high reaction temperature and indicates a σ-bond metathesis pathway.
- A Boron–Boron Double Transborylation Strategy for the Synthesis of gem-Diborylalkanes,
Jamie H. Docherty, Kieran Nicholson, Andrew P. Dominey, Stephen P. Thomas,
ACS Catal. 2020.
https://doi.org/10.1021/acscatal.0c00869