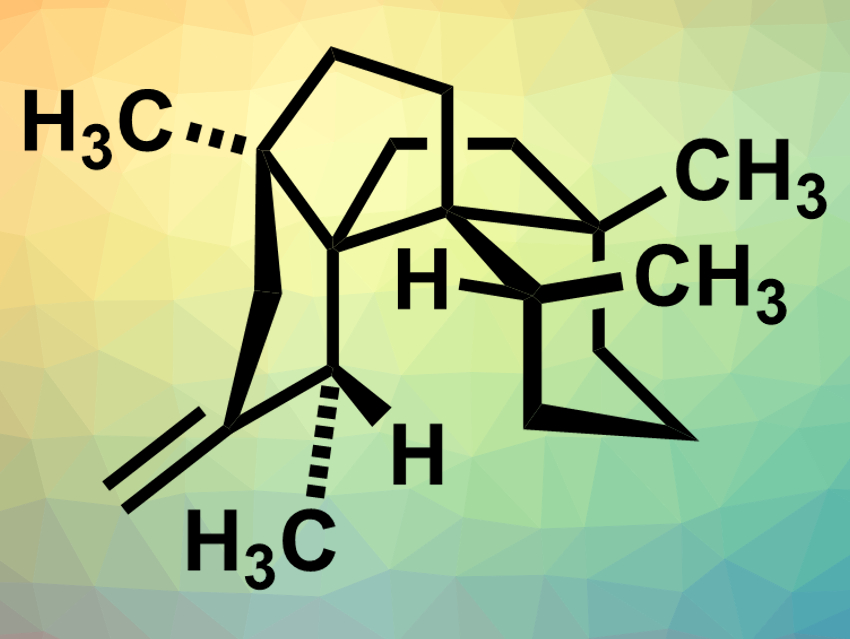
Polyquinanes are polycyclic organic compounds consisting of fused five-membered rings. This structure is found in many natural products, for example, in waihoensene (pictured), which was isolated from the plant Podocarpus totara var. waihoensis in New Zealand. This compound has a complex structure with six contiguous stereogenic centers—four of them quarternary carbon atoms. This makes it challenging to synthesize waihoensene in an enantioselective manner.
Jun Huang, Peking University Shenzhen Graduate School, China, Zhen Yang, Peking University Shenzhen Graduate School and Peking University, Beijing, and colleagues have performed the first asymmetric total synthesis of (+)-waihoensene. The team started by preparing a diyne-substituted cyclohexanone in an enantioselective way. This compound was converted to a polycyclic diketone intermediate that contains all four quarternary stereogenic centers via a Conia-ene type cyclization, a Pauson–Khand reaction, and a nickel-catalyzed methylation. The resulting intermediate was methylated twice and the olefin group was created using a Wittig reaction to obtain the desired product.
Overall, the synthesis involved 15 steps and gave a yield of 3.8 %. According to the researchers, the synthesis strategy could also be useful for the preparation of other complex natural products.
- Asymmetric Total Synthesis of (+)-Waihoensene,
Yongzheng Qu, Zheyuan Wang, Zhongchao Zhang, Wendou Zhang, Jun Huang, Zhen Yang,
J. Am. Chem. Soc. 2020.
https://doi.org/10.1021/jacs.0c02143