Fluorophores, i.e., compounds that re-emit light upon light excitation, are useful as tracers and probes, especially in biochemistry and medicinal chemistry. So-called “turn-on” or “caged” fluorophores are inactive until a trigger releases the active version of the compound. This trigger can, e.g., be visible light, resulting in a photoactivatable fluorophore. This allows researchers to control when and where the fluorescence signal is activated.
Han Xiao, Rice University, Houston, TX, USA, and colleagues have used tetrazine as phototrigger for photoactivatable fluorophores. The team bound a methyl-substituted tetrazine group to different fluorophores, such as BODIPY (boron-dipyrromethene), coumarin, or rhodamine dyes. In the resulting conjugates, the tetrazine acts as a quencher that turns off the fluorescence of the dyes.
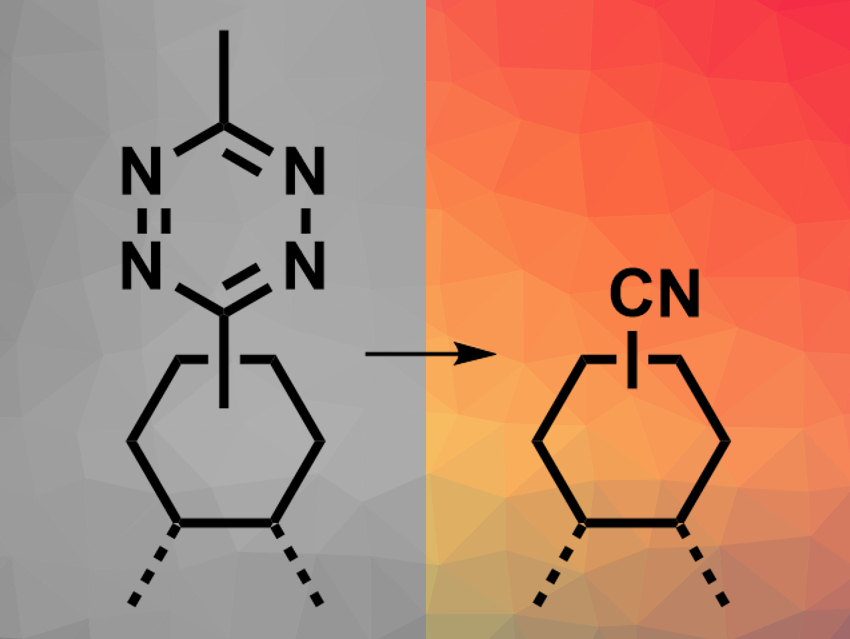
When the tetrazine–dye conjugates are irradiated with light, the tetrazine group decomposes (pictured). This reaction gives two nitriles, one that remains bound to the dye and one equivalent of acetonitrile, as well as molecular nitrogen. The quenching effect is, thus, removed and the fluorescence is turned on. The developed photoactivatable probes can, for example, be used for the imaging of cell organelles the high-resolution imaging of proteins.
- Tetrazine as a General Phototrigger to Turn on Fluorophores,
Axel Loredo, Juan Tang, Lushun Wang, Kuan-Lin Wu, Zang Peng, Han Xiao,
Chem. Sci. 2020.
https://doi.org/10.1039/d0sc01009j