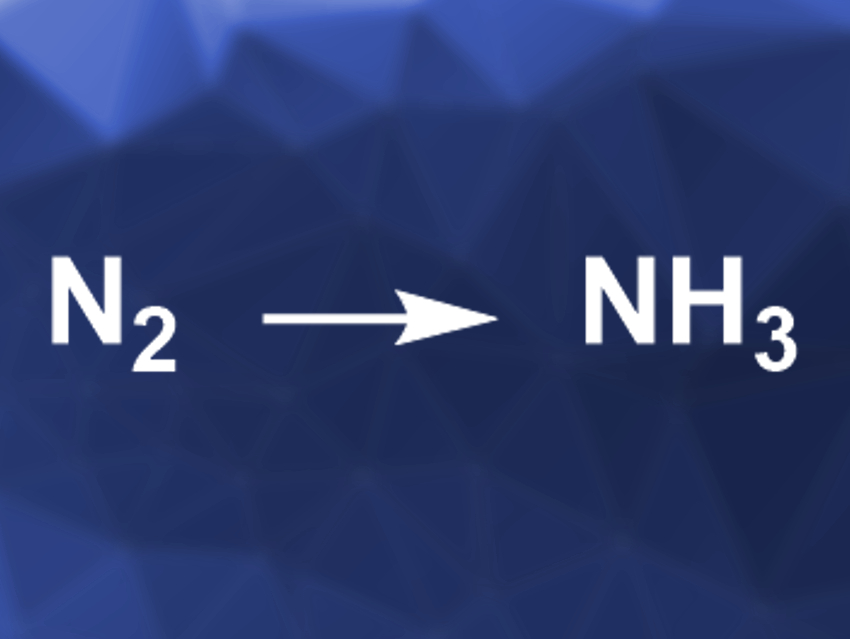
Ammonia is an important product of the chemical industry, especially for the preparation of fertilizer. It is usually made via the Haber-Bosch process, which requires high temperatures and pressures to convert N2 and H2 to NH3. This process has a very high energy consumption, and alternatives would be useful. Photocatalytic nitrogen fixation, e.g., could be promising, but so far, catalysts for this approach do not provide sufficient activity.
Yasuhiro Shiraishi, Osaka University, Toyonaka, Japan, and colleagues have used bismuth oxychloride with surface oxygen vacancies (BiOCl-OVs) as a photocatalyst for the production of NH3 from N2 in chloride-containing water under irradiation with ultraviolet light. The semiconductor catalyst was prepared using a solvothermal method starting from Bi(NO3)3·5 H2O and KCl in ethylene glycol.
In pure water, the catalyst does not efficiently promote nitrogen fixation. This is due to chloride removal during the reaction, which inactivates the catalyst. When chloride ions are present in an acidic reaction solution, this deactivation is avoided and the catalyst efficiently produces NH3 under a flow of N2. Overall, the work provides a new concept for nitrogen fixation based on a low-cost catalyst and, e.g., seawater, which contains sufficient amounts of chloride.
- Photocatalytic Dinitrogen Fixation with Water on Bismuth Oxychloride in Chloride Solutions for Solar-to-Chemical Energy Conversion,
Yasuhiro Shiraishi, Masaki Hashimoto, Kiyomichi Chishiro, Kenta Moriyama, Shunsuke Tanaka, Takayuki Hirai,
J. Am. Chem. Soc. 2020.
https://doi.org/10.1021/jacs.0c01683