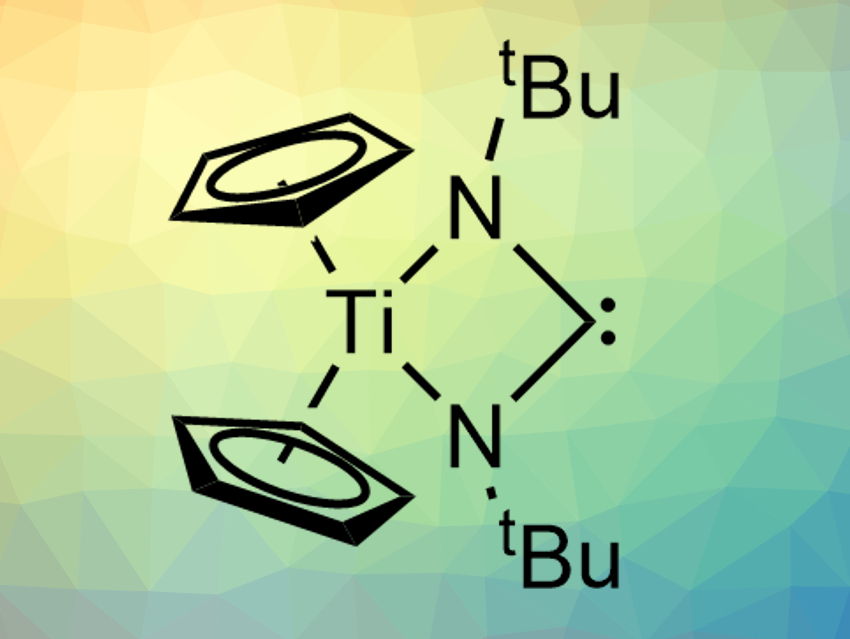
Heterocumulenes are molecules with multiple double bonds next to each other, connecting at least one heteroatom with carbon atoms. CO2, for example, can be considered a heterocumulene. Compounds such as CO2 can bind to transition metals in a variety of binding modes. However, there had been no examples of monometallic complexes with a heterocumulene of the type ECE (E = heteroatom) in a bidentate κ2-bonding mode so far.
Christophe Copéret, Swiss Federal Institute of Technology (ETH) Zurich, Switzerland, Jason D. Goodpaster, Ian A. Tonks, University of Minnesota − Twin Cities, Minneapolis, USA, and colleagues have synthesized such a complex, i.e., Cp2Ti(κ2–tBuNCNtBu) (pictured). The team prepared the complex from the precursor Cp2Ti(η2-BTMSA) (BTMSA = bis(trimethylsilylacetylene) by reacting it with the heterocumulene tBuN=C=NtBu, which is a 1,3-disubstituted carbodiimide, in pentane at 25 °C.
The resulting complex was characterized using X-ray crystallography and NMR spectroscopy, and its bond situation was investigated using quantum-chemical calculations. The team found that Cp2Ti(κ2–tBuNCNtBu) is diamagnetic and forms a free carbene, which is stabilized by a Ti-NCN π-interaction in the metallacycle. According to the researchers, the work could be a basis for further investigations of metal–heterocumulene bonding.
- Cp2Ti(κ2–tBuNCNtBu): A Complex with an Unusual κ2 Coordination Mode of a Heterocumulene Featuring a Free Carbene,
Evan P. Beaumier, Christopher P. Gordon, Robin P. Harkins, Meghan E. McGreal, Xuelan Wen, Christophe Copéret, Jason D. Goodpaster, Ian A. Tonks,
J. Am. Chem. Soc. 2020.
https://doi.org/10.1021/jacs.0c02487