Carbon monoxide (CO) can act as a biological signaling molecule. This can be useful for biomedical applications, for example, to promote wound healing. Since CO gas is difficult to handle and toxic at high levels, such applications rely on the controlled delivery of small amounts of CO from CO-releasing molecules (CORMs). These can be either metal-carbonyl-based or metal-free compounds, such as 3-hydroxyflavone derivatives. This type of CORM can, e.g., be triggered by light to release CO.
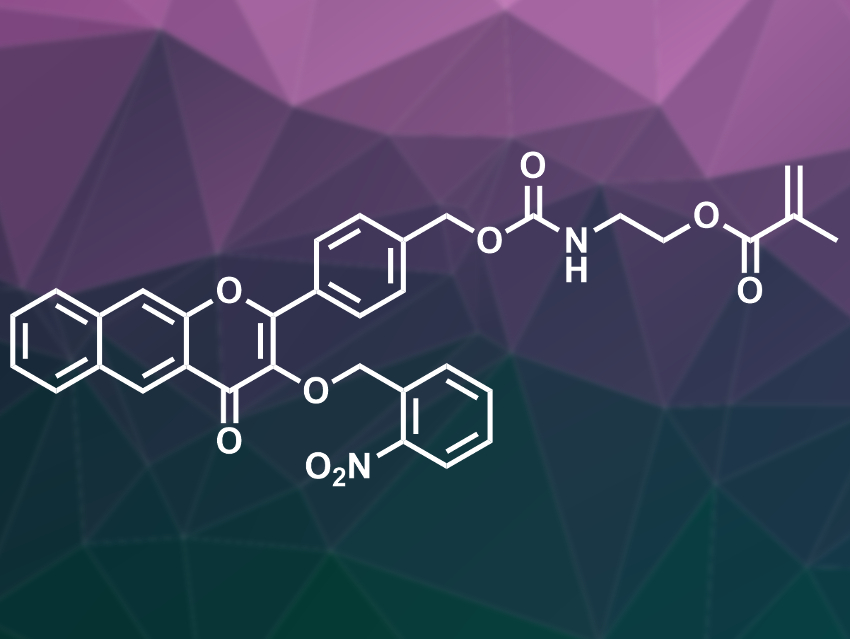
Bin Zheng, Hefei Normal University, China, Jinming Hu, University of Science and Technology of China, Hefei, and colleagues have synthesized a polymerizable molecule that contains both a photoresponsive o-nitrobenzyl ether and a 3-hydroxyflavone derivative, 2-((((4-(3-((2-nitrobenzyl)oxy)-4-oxo-4H-benzo[g]chromen-2-yl)benzyl)oxy)carbonyl)amino)ethyl methacrylate (FNM, pictured). This monomer was polymerized via a RAFT (reversible addition-fragmentation chain transfer) polymerization using a poly(ethylene oxide) (PEO)-based agent. This gives a block copolymer consisting of PEO and poly-FNM. In an aqueous solution, this amphiphilic copolymer self-arranges into micelles and, thus, can be easily dispersed.
When the copolymer is triggered by light, the 2-nitrobenzyl group is removed first. The product is still light-sensitive, degrades further, and releases CO. During this process, the compound’s fluorescence changes from blue to red to non-emissive, which allowed the researchers to track the CO release. The team tested the effects of the copolymer in a mouse model and found that it promotes skin wound healing when irradiated with light.
- Metal-Free Carbon Monoxide-Releasing Micelles Undergo Tandem Photochemical Reactions for Cutaneous Wound Healing,
Jian Cheng, Bin Zheng, Sheng Cheng, Guoying Zhang, Jinming Hu,
Chem. Sci. 2020.
https://doi.org/10.1039/d0sc00135j