Cytochrome P450 monooxygenases are enzymes that can catalyze a wide variety of reactions. They have a remarkable substrate promiscuity and can catalyze both oxidative and non-oxidative reactions. P450 enzymes, thus, have potential for use in biotechnological applications and pharmaceutical manufacturing. Efforts have been made to engineer P450-based systems to create biocatalysts for difficult biotransformation procedures.
Most P450 enzyme systems require two parts: a heme domain and an added redox partner. The former is the substrate-binding site and the latter helps to transfer electrons from cofactors (reduced pyridine nucleotides such as NADPH or NADH) to the substrate. Functional P450 systems, thus, require the co-expression of compatible redox partners. Self-sufficient P450 enzymes, in contrast, contain the heme domain and the redox partners in a single polypeptide chain. Thus, they are particularly promising for biotechnological applications. A large number of crystal structures of separate heme domains and redox partners are known. However, no structural information of a full-length self-sufficient P450 had been reported so far.
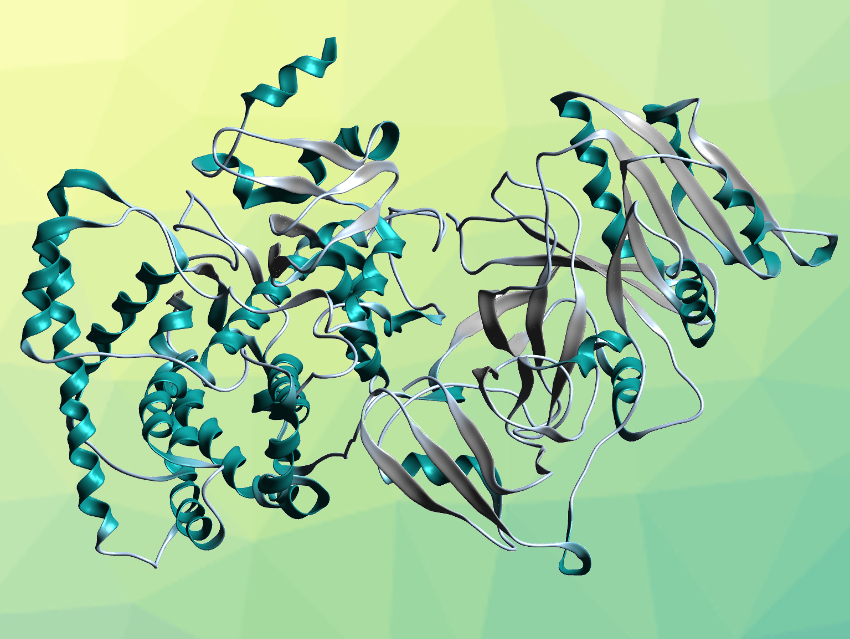
Rey-Ting Guo, Chun-Chi Chen, Hubei University, Wuhan, China, and colleagues have determined a high-resolution crystal structure of full-length CYP116B46 (pictured), a self-sufficient P450. The enzyme was expressed in Escherichia coli, purified, and crystallized. The crystal structure was then determined using X-ray diffraction with a resolution of 2.13 Å. The polypeptide folds into a reductase domain (pictured on the right), a ferredoxin domain (pictured in the lower center), and a heme domain (pictured on the left). These domains contain a flavin mononucleotide (FMN), a binuclear Fe-S cluster of the type [2Fe-2S], and a heme unit, respectively.
The domain arrangement aligns well with the direction of electron transfer. It indicates a pathway from FMN via the [2Fe-2S] cluster to the heme unit. The locations of FMN and [2Fe-2S] are close enough to allow direct electron transfer. The team examined protein residues along the path from the [2Fe-2S] cluster to the heme unit and found several polar residues that could contribute to electron transport. Overall, the work provides insights into the mechanism of action of self-sufficient P450s that could be useful as guidance for the development of P450-based applications.
- Structural insight into the electron transfer pathway of a self-sufficient P450 monooxygenase,
Lilan Zhang, Zhenzhen Xie, Ziwei Liu, Shuyu Zhou, Lixin Ma, Weidong Liu, Jian-Wen Huang, Tzu-Ping Ko, Xiuqin Li, Yuechan Hu, Jian Min, Xuejing Yu, Rey-Ting Guo, Chun-Chi Chen,
Nat Commun. 2020.
https://doi.org/10.1038/s41467-020-16500-5