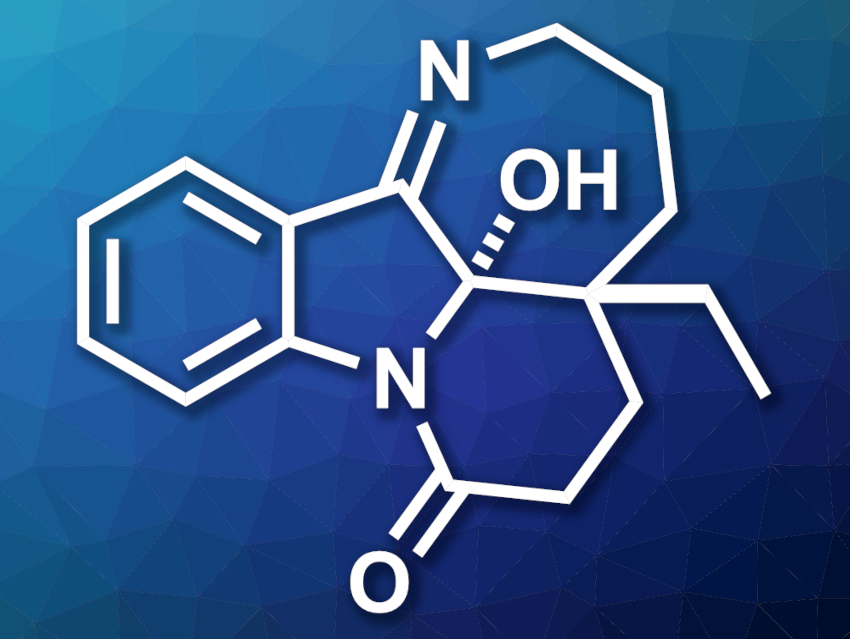
Mersicarpine (pictured) is an alkaloid first isolated from Kopsia plants. This natural product has shown bioactivity against some human cancer cell lines and is an interesting target for total synthesis. Existing synthetic approaches to mersicarpine are generally based on the oxidation of indole precursors.
Malte Brasholz, University of Rostock and Leibniz-Institut für Katalyse e.V., Rostock, Germany, and colleagues have developed a short total synthesis of (±)-mersicarpine that involves a visible-light-induced cascade photooxygenation. The team started from cyclohexanone-4-carboxylate, which was converted to a 3,3-disubstituted tetrahydrocarbazole, a type of indole-containing precursor, in eight steps. The tetrahydrocarbazole intermediate was subjected to a hydrogenolysis and a protection step. Then, the resulting 3-(aminopropyl)-substituted tetrahydrocarbazole was oxidized in the key step, a visible-light-mediated cascade photooxygenation using rose bengal as a sensitizer, performed under an atmosphere of O2 and green LED irradiation.
This cascade reaction gives a hydroxytetrahydropyrido[1,2-a]indole dione, which contains three new oxygen functionalities. Finally, the remaining protection group was removed and the seven-membered ring was closed to give (±)-mersicarpine. The product was obtained in an overall yield of 12 % over 13 steps.
- A short total synthesis of (±)-mersicarpine via visible light-induced cascade photooxygenation,
Mario Frahm, Alice Voss, Malte Brasholz,
Chem. Commun. 2022.
https://doi.org/10.1039/d2cc01316a