Water splitting driven by sunlight could be used to provide hydrogen as a clean, sustainable fuel. This process requires efficient photocatalysts. These catalysts need to be able to provide a separation of charges, or a separation of redox sites, to promote the splitting of water into hydrogen and oxygen. This separation can, for example, be achieved at the interface between the different crystal facets of catalysts such as bismuth vanadate (BiVO4). However, this effect of the different crystal facets in BiVO4 is fairly small and could be optimized to provide better catalysts.
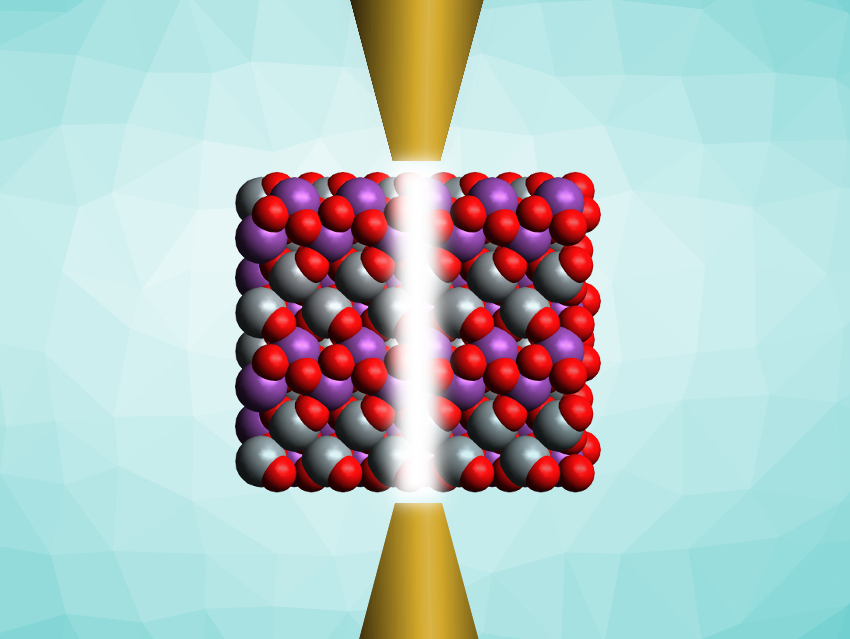
Changhua Wang, Xintong Zhang, Northeast Normal University, Changchun, China, and colleagues have used a plasma-based surface treatment to improve the catalytic activity of decahedral BiVO4 crystals. The team synthesized BiVO4 from Bi(NO3)3·5H2O and NH4VO3 in a nitric acid solution. The resulting crystalline product was added to a KCl solution in a reactor with two opposing tungsten electrodes. A high-voltage pulse was then applied to create a plasma in the solution between the electrodes. This plasma etching treatment induces defects in the surface of the BiVO4 crystals.
The team found that the plasma-treated crystals perform better than the untreated parent compound as water-splitting catalysts, while their overall decahedral shape remained unchanged. The team used Raman spectroscopy, X-ray photoelectron spectroscopy (XPS), and X-ray fluorescence (XRF) to investigate the structure of the treated BiVO4. They found that the etching introduces vanadium vacancies on the surface of the BiVO4 crystals, especially on the {110} facet. These vacancies act as “electron traps” and improve the charge separation at the different crystal facets—and thus, the catalytic activity.
- Solution plasma boosts facet-dependent photoactivity of decahedral BiVO4,
Guangshun Che, Dandan Wang, Changhua Wang, Fei Yu, Dashuai Li, Norihiro Suzuki, Chiaki Terashima, Akira Fujishima, Yichun Liu, Xintong Zhang,
Chem. Eng. J. 2020, 397, 125381.
https://doi.org/10.1016/j.cej.2020.125381