Drugs such as naloxone can block the effects of opioids, e.g., heroin. They can save lives as an emergency drug against opioid overdoses. The opioid crisis has caused a rise in demand—and higher prices—for this type of drug. More efficient synthetic approaches could help to solve this issue.
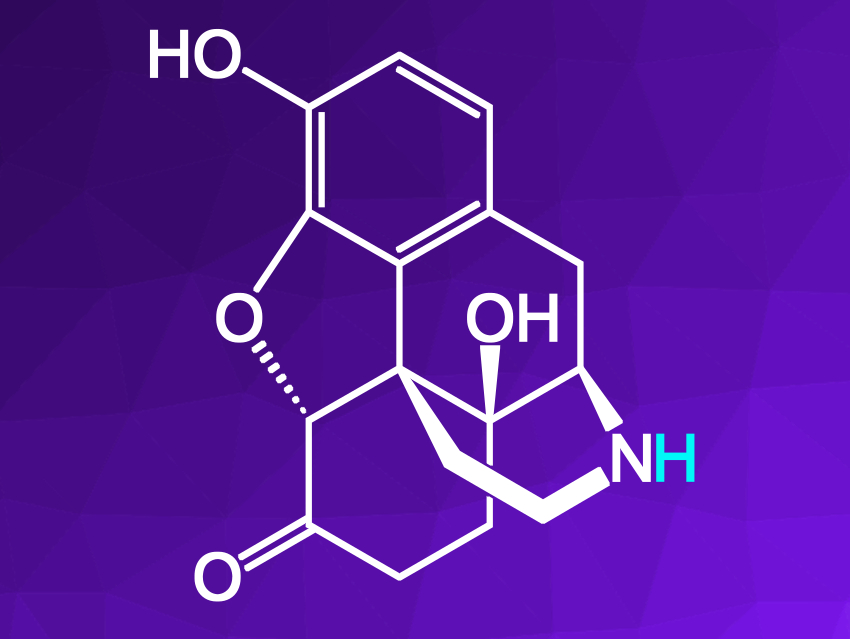
Naloxone and the related drug naltrexone share a 14-hydroxy morphinan skeleton. These drugs are prepared from a complex morphine precursor by N-demethylation to give the corresponding nor-opioid (pictured), followed by an N-alkylation to give the desired drugs. The N-demethylation is the most challenging step in the synthesis, and usually requires excess amounts of harmful reagents, such as cyanogen bromide.
Gabriel Glotz, C. Oliver Kappe, and David Cantillo, University of Graz and Research Center Pharmaceutical Engineering GmbH (RCPE), both Graz, Austria, have developed a reagent- and catalyst-free electrochemical procedure for this N-demethylation reaction. The team electrolyzed precursors such as oxycodone or 3,14-diacetylmorphinone in an undivided cell with graphite as the anode and stainless steel as the cathode at room temperature. A 4:1 mixture of acetonitrile and methanol was used as the solvent, together with Et4NBF4 as a supporting electrolyte.
Depending on the precursor, this electrochemical process leads to a cyclization, forming oxazolidines with neighboring hydroxy groups, or to an O,N-acyl transfer with neighboring acetyl groups. The resulting intermediates can easily be hydrolyzed under acidic conditions to give the desired N-demethylated product in high yields. According to the researchers, the reaction proceeds via a two-electron anodic oxidation of the tertiary amine precursor, which creates an iminium ion that rapidly undergoes cyclization or O,N-acyl transfer. The team also transferred this approach to a flow electrolysis cell, which could allow its scale-up.
- Electrochemical N-Demethylation of 14-Hydroxy Morphinans – Sustainable Access towards Opioid Antagonists,
David Cantillo, C. Oliver Kappe, Gabriel Glotz,
ChemRxiv 2020.
https://doi.org/10.26434/chemrxiv.12458573.v1The research has been published as a preprint and has not yet been peer-reviewed.