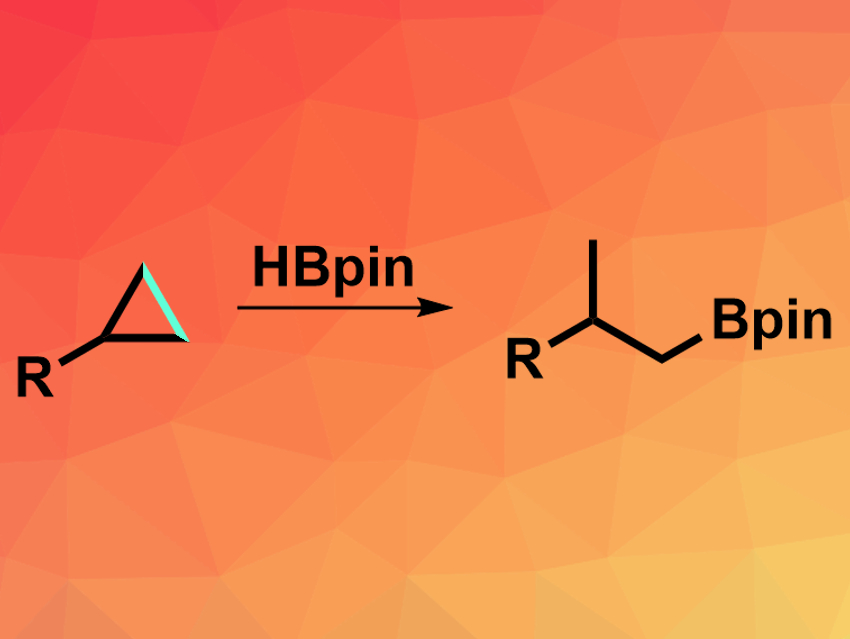
Hydroborations are very common reactions in organic synthesis. In this transformation, electrophilic boranes are added to alkenes to give alkylboranes. This reaction needs the reactivity of the C=C π-bonds and generally cannot be used on the C–C σ-bonds of alkanes. However, such a hydroboration of alkanes could allow the preparation of useful new alkylboranes that are different from the “traditional” hydroboration products. Alkylboranes can be further functionalized to give a variety of organic compounds.
Daisuke Yokogawa, Nagoya University, Japan, Junichiro Yamaguchi, Waseda University, Tokyo, Japan, and colleagues have developed the first general hydroboration of cyclopropanes (pictured above). The team reacted a variety of substituted cyclopropanes with pinacolborane (HBpin)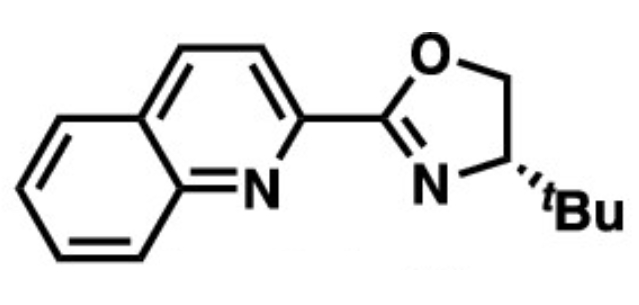 in the presence of [Ir(OMe)(cod)]2 (cod = 1,5-cyclooctadiene) as a catalyst and t-BuQuinox (pictured on the right) as a ligand in tetrahydrofuran (THF) at 80 °C. The desired hydroboration products were obtained in moderate to good yields.
in the presence of [Ir(OMe)(cod)]2 (cod = 1,5-cyclooctadiene) as a catalyst and t-BuQuinox (pictured on the right) as a ligand in tetrahydrofuran (THF) at 80 °C. The desired hydroboration products were obtained in moderate to good yields.
The team found that the t-BuQuinox ligand was essential to achieve selective C–C bond activation. With other types of ligands, the reaction gives borylated cyclopropanes via the activation of a C–H bond. The team attributes this effect to a difference in steric repulsion between ligand and reactant, which favors the C–C activation over the C–H activation. The obtained hydroboration products can, for example, be oxidized to give the corresponding alcohol or used as a partner in coupling reactions.
- σ-Bond Hydroboration of Cyclopropanes,
Hiroki Kondo, Shin Miyamura, Kaoru Matsushita, Hiroki Kato, Chisa Kobayashi, Arifin, Kenichiro Itami, Daisuke Yokogawa, Junichiro Yamaguchi,
J. Am. Chem. Soc. 2020.
https://doi.org/10.1021/jacs.0c05213