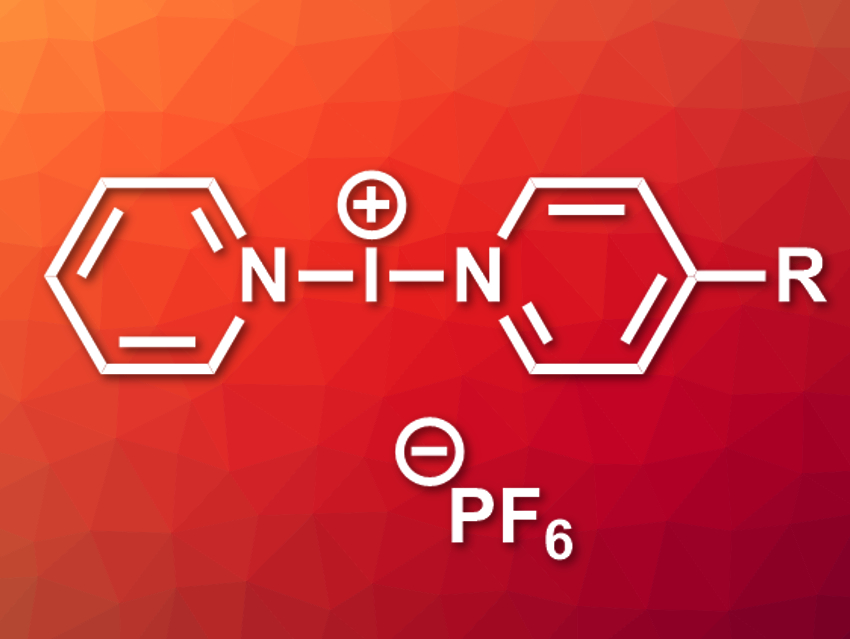
Halonium ions, i.e., X+ (X = Cl, Br, I), are strong halogen-bond (XB) donors. They have useful reactivities for organic synthesis. Certain iodonium compounds can, e.g., be used as mild iodinating agents. Barluenga’s reagent [I(py)2][BF4] (py = pyridine) is a well-known example. Extending this type of symmetric, homoleptic iodonium complex to asymmetric complexes with two different ligands could be useful for asymmetric syntheses.
Jas S. Ward, Kari Rissanen, University of Jyväskylä, Finland, and colleagues have synthesized two asymmetric halogen-bonded iodonium complexes, [I(py)(4-DMAP)]PF6 and [I(py)(4-Etpy)]PF6 (pictured, R = NMe2 or Et, respectively). The team first prepared the corresponding silver(I) complexes by combining AgPF6, pyridine, and the respective substituted pyridine derivative in a 1:1:1 ratio in CH2Cl2. The resulting Ag+-precursors were reacted with elemental iodine in CH2Cl2 to give the desired iodonium complexes via a cation exchange.
The silver(I) precursors and iodonium complexes were characterized using NMR spectroscopy and single-crystal X-ray crystallography. The team found an unusually short I+–I+ distance in the solid state for the 4-DMAP complex. This close contact was analyzed using density functional theory (DFT) calculations, which confirmed the presence of an attractive interaction between the I+ centers.
- Asymmetric [N-I-N]+ Halonium Complexes,
Jas S. Ward, Giorgia Fiorini, Antonio Frontera, Kari Rissanen,
Chem. Commun. 2020.
https://doi.org/10.1039/d0cc02758h