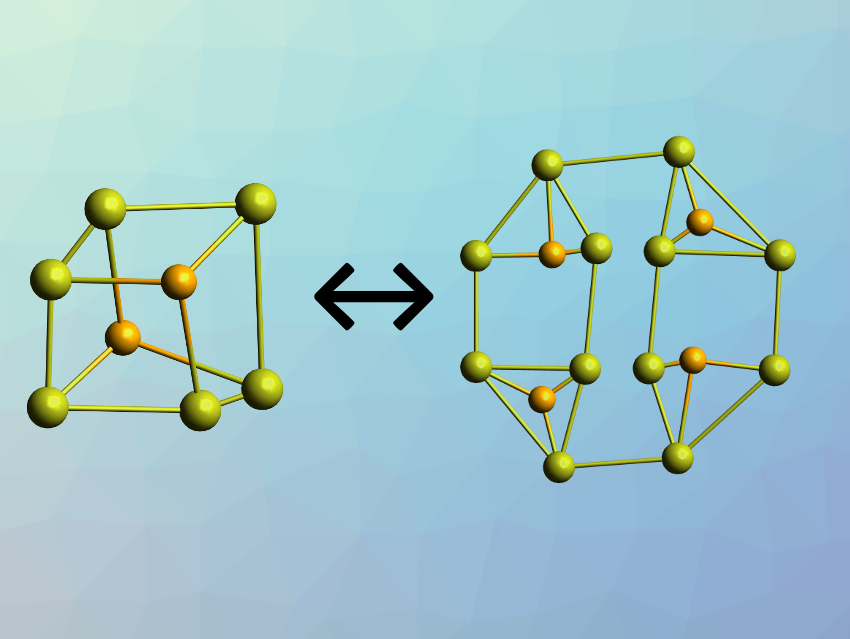
Chemical systems that can change their structure upon external stimuli, i.e., undergo so-called supramolecular transformations, have are interesting research subjects. Such transformations are usually based on the manipulation of non-covalent interactions. Metal complexes are interesting in this context due to their reversible metal–ligand bonds. Polynuclear gold(I) complexes, for example, can have a variety of conformations, held together by both metal–ligand bonds and gold(I)–gold(I) interactions.
Vivian Wing-Wah Yam, The University of Hong Kong, Hong Kong SAR, China, and colleagues have discovered a concentration-dependent and solvation-dependent reversible cluster-to-cluster transformation between a hexanuclear and a dodecanuclear gold(I) sulfido complex (pictured schematically, ligands omitted for clarity). The team used bis(diphenylphosphino)amine ligands of the type Ph2P–N(R)–PPh2 (R = Et, Me, or H) to prepare polynuclear gold(I) sulfido complexes. The products were characterized using NMR spectroscopy, high-resolution electrospray-ionization mass spectrometry (HR-ESI-MS) analysis, and single-crystal X-ray diffraction.
The researchers obtained a decanuclear (Au10) complex when using the Et-substituted ligand, a mixture of Au10 and the Au12 complexes with the Me-substituted ligand, and a dodecanuclear (Au12) complex for R = H. The team concluded that the steric effect of the ligands dictates the nuclearity of the complexes. In addition, for the complex with the Me-substituted ligand, the team observed an unprecedented reversible concentration-dependent transformation: The Au12 complex can be converted to an Au6 complex by diluting the solution, and converted back to the Au12 species when the concentration is increased. This conversion can also be triggered by changing the polarity of the solvent. According to the researchers, the work improves the understanding of supramolecular transformations in polynuclear gold(I) systems under the application of external stimuli.
- Concentration- and Solvation-Induced Reversible Structural Transformation and Assembly of Polynuclear Gold(I) Sulfido Complexes,
Liang-Liang Yan, Liao-Yuan Yao, Vivian Wing-Wah Yam,
J. Am. Chem. Soc. 2020.
https://doi.org/10.1021/jacs.0c04677