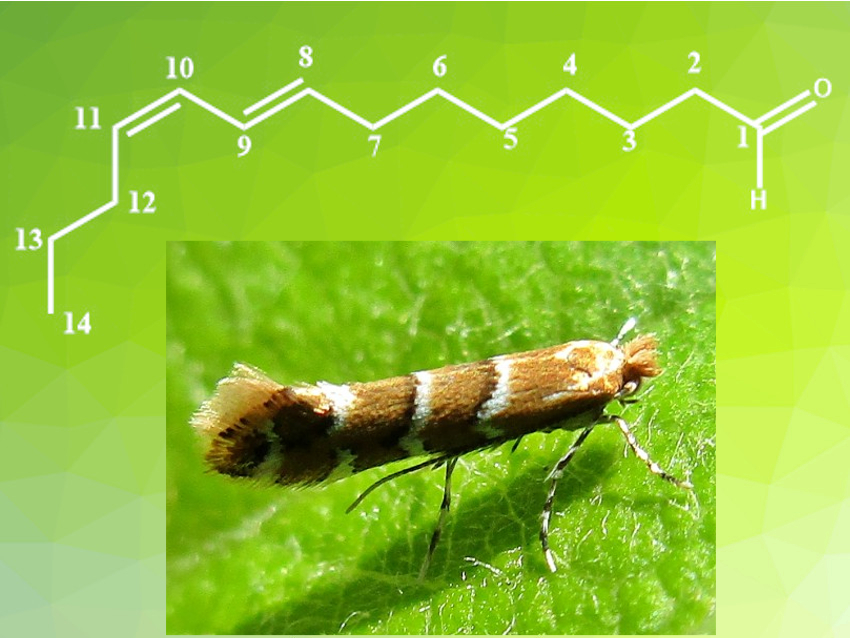
The horse-chestnut leaf miner is a moth species that causes serious damage to horse-chestnut trees in Europe. The use of pesticides to control this insect can also harm non-target species and pose dangers to human health. This is especially problematic in the case of horse-chestnut trees, because they are often grown in residential areas. The use of sex pheromones for pest control is a sustainable alternative to pesticides. Pheromones can be used to attract and/or trap the insects. The effects of these molecules are species-specific, and they are not dangerous to human health.
Eric Gayon, M2i Development, Lacq, Guillaume Lefèvre, Chimie ParisTech, PSL University, Paris, France, and colleagues have developed a scalable six-step synthesis route for the horse-chestnut leaf miner sex pheromone (8E,10Z)-tetradeca-8,10-dienal (pictured) with an overall yield of 40 %. The product was prepared in two parts, which were then coupled to form the C7–C8 linkage. The C1–C7-fragment was prepared from diethyl pimelate, which was reduced using sodium (bis(2-methoxyethoxy)aluminum hydride (Red-Al). The resulting diol was monochlorinated using aqueous hydrochloric acid in toluene and then converted to the corresponding Grignard reagent by addition of n-BuMgCl in THF. The second fragment, (1E-3Z)-dienol phosphate, was prepared from trans-hept-2-enal, which was first treated with t-BuOK and then reacted with diethyl chlorophosphite.
The two fragments were coupled in an iron-catalyzed Kumada coupling reaction, using the easily available and inexpensive iron complex Fe(acac)3. The resulting alcohol was oxidized to the desired aldehyde using iodobenzene diacetate in the presence of 2,2,6,6-tetramethylpiperidin-1-oxyl (TEMPO) to give the pheromone in 90 % diastereomeric purity.
As an abundant metal with low toxicity, iron is more convenient and environmentally friendly for large-scale reactions than metals such as palladium and nickel that are more commonly used for C–C cross-couplings. The reaction proceeds without additional ligands or additives, which minimizes waste and makes the reaction safer and more cost-efficient. The researchers were able to synthesize 40 g of the pheromone in one batch. Due to the high effectiveness of the compound, this amount is sufficient for the treatment of an area of ca. 8 km2.
- Short and Easily Scalable Synthesis of the Sex Pheromone of the Horse-Chestnut Leaf Miner (Cameraria ohridella) Relying on a Key Ligand- and Additive-Free Iron-Catalyzed Cross-Coupling,
Pablo Chourreu, Olivier Guerret, Loïc Guillonneau, Eric Gayon, Guillaume Lefèvre,
Org. Process Res. Dev. 2020.
https://doi.org/10.1021/acs.oprd.0c00191