Porous materials such metal–organic frameworks (MOFs) and covalent organic frameworks (COFs) can be used in sensing applications. Hydrogen-bonded organic frameworks (HOFs) are less well-studied porous materials. They have advantages in ease of processing over other porous materials and could be recovered and reused by simple recrystallization. HOFs for fluorescent sensing applications need to have suitable pores for specific and selective recognition and a tight interaction between the analytes and the HOF to change the fluorescent properties. Most often, changes in luminescence intensity (turn-down or turn-up) are used as the measurable response. Turn-up fluorescent sensing is more sensitive and reliable, but more difficult to realize.
Zhangjing Zhang, Fujian Normal University, Fuzhou, China, Banglin Chen, University of Texas at San Antonio, USA, and colleagues have developed a HOF for the highly efficient turn-up fluorescent sensing of aniline. Aniline is a toxic pollutant. The framework, called HOF-20, was prepared from 5-(2,6-bis(4-carboxyphenyl)pyridin-4-yl)isophthalic acid (H4BCPIA), an aromatic building block with four carboxylic acid groups. It was obtained in the form of needle-like crystals by slowly evaporating an N,N-dimethylformamide (DMF) solution of H4BCPIA.
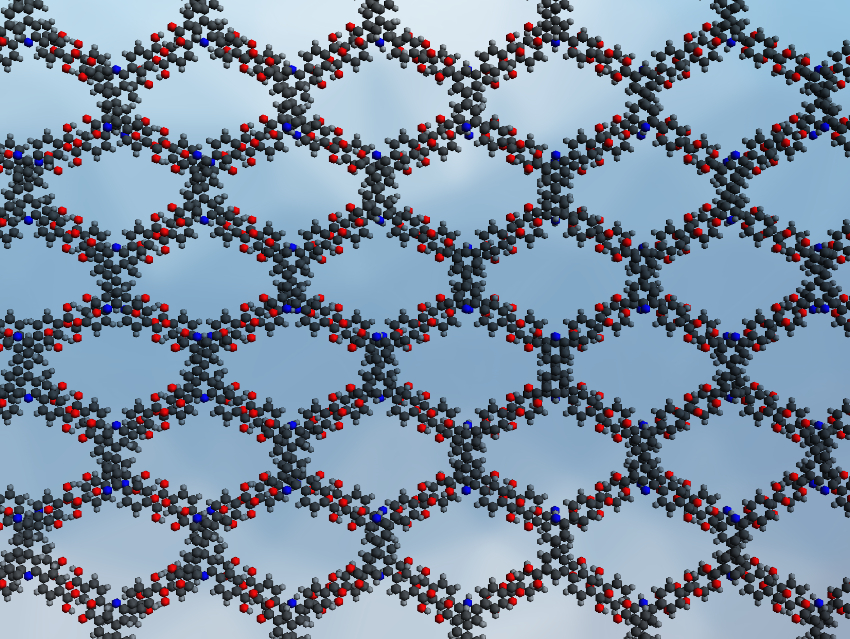
X-ray crystallography showed that the HOF forms a porous framework (pictured). Each building block is connected to four neighbors through hydrogen bonds between the carboxylic acid groups. The team investigated the fluorescence sensing ability of HOF-20 toward aniline in aqueous solutions. They found that the fluorescence of HOF-20 was significantly enhanced in the presence of aniline. This enhancement was selective for aniline and did not occur when similar benzene derivatives were used. After sensing, the HOF could be regenerated by simply washing it with water. According to the researchers, the fluorescent turn-up is due to trapped aniline molecules restricting the rotation of the aromatic rings in the HOF (through hydrogen bonding and π–π interactions). This can reduce nonradiative decay pathways, and thus, enhance fluorescence.
- Microporous Hydrogen-Bonded Organic Framework for Highly Efficient Turn-Up Fluorescent Sensing of Aniline,
Bin Wang, Ru He, Lin-Hua Xie, Zu-Jin Lin, Xin Zhang, Jing Wang, Hongliang Huang, Zhangjing Zhang, Kirk S. Schanze, Jian Zhang, Shengchang Xiang, Banglin Chen,
J. Am. Chem. Soc. 2020.
https://doi.org/10.1021/jacs.0c05277