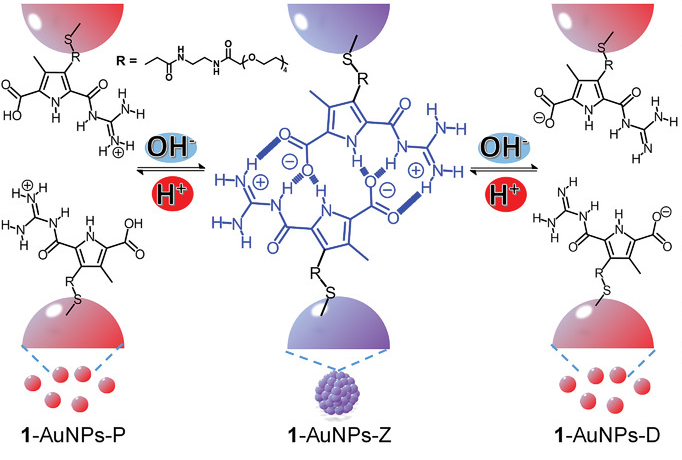
The pH-induced, reversible self-assembly (PIRSA) of nanoparticles (NPs) can be useful, e.g., in imaging or drug-delivery applications. Usually, such an effect is achieved using pH-responsive ligands on the nanoparticles. Most often, this allows NPs to assemble in one specific pH range and disassemble in another pH range (mono-PIRSA). By using ligands with multiple possible protonation states, multi‐pH responsive systems can be built. These systems can assemble or disassemble at more than one pH “border”. Zwitterionic ligands could be used for this. However, only mono-PIRSA had been achieved in metal NPs functionalized with small zwitterionic ligands so far.
Huibin He, Jochen Niemeyer, University of Duisburg‐Essen, Germany, and colleagues have prepared gold nanoparticles (Au NPs) that show a dual-PIRSA, i.e., the NPs assemble at neutral pH, and disassemble at both acidic or basic pH values (pictured). The team used guanidiniocarbonyl pyrrole carboxylate zwitterion (GCPZ) ligands to achieve this effect. The pH-responsive system was created by mixing Au NPs with the ligand and dispersing the functionalized NPs in a DMSO/water mixture.
GCPZs can form stable dimers held together by hydrogen bonds (pictured below in blue). This causes self-assembly of the nanoparticles at neutral pH. At basic pH, the ligands are deprotonated and the nanoparticles separate (pictured below on the right). When an acid, e.g., HCl is added, the ligands are protonated, which also leads to disassembly (pictured below on the left). According to the researchers, a combination of electrostatic and hydrophobic effects is probably responsible for the observed self-assembly behavior.
- Dual pH‐Induced Reversible Self‐Assembly of Gold Nanoparticles by Surface Functionalization with Zwitterionic Ligands,
Huibin He, Jan‐Erik Ostwaldt, Christoph Hirschhäuser, Carsten Schmuck, Jochen Niemeyer,
Small 2020.
https://doi.org/10.1002/smll.202001044