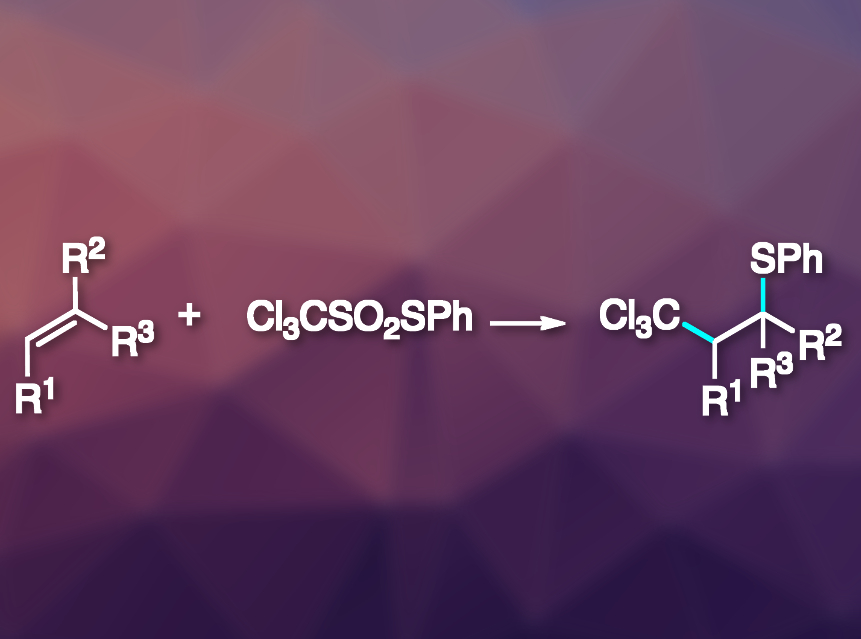
Mild and selective carbon–carbon bond forming reactions via radical pathways, such as atom- or group-transfer radical additions (ATRA or GTRA), are atom-economic and efficient. However, their substrate scope is limited by the need for a relatively weak C–X bond (X = e.g., Cl, Br, I, SeR). This problem can be avoided by introducing an –SO2– unit as a leaving group between C and X, leading to desulfitative group- or atom-transfer radical additions.
Philippe Renaud, University of Bern, Switzerland, and colleagues have developed an efficient method for the thioalkylation of alkenes via a radical desulfitative sulfur-group transfer (pictured). The team prepared S-phenyl trichloromethanethiosulfonate (Cl3CSO2SPh) from commercially available Cl3CSO2Cl by reacting it first with sodium sulfite and then with benzenesulfenyl chloride (PhSCl). S-Ethyl trichloromethanethiosulfonate (Cl3CSO2SEt) was prepared analogously. The researchers used these reagents, together with dilauryl peroxide (DLP) as an initiator, to perform thioalkylations of a variety of alkenes under sun-lamp irradiation.
The desired products were obtained in good to excellent yields. The team also extended the reaction to ethoxycarbonylmethyl radical sources instead of trichloromethyl species. According to the researchers, it might be possible to use the same approach with many other electrophilic radicals.
- Desulfitative Thioalkylation of Alkenes,
Lidong Cao, Ciril Jimeno, Philippe Renaud,
Adv. Synth. Catal. 2020.
https://doi.org/10.1002/adsc.202000657