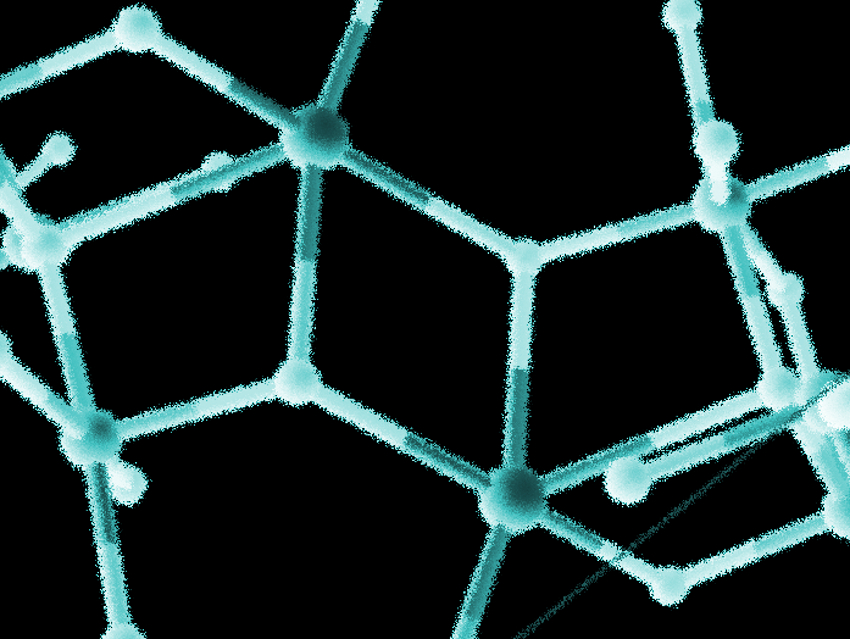
Manganese–calcium clusters play an important role in biochemistry, i.e., in the active center of photosystem II (PSII), which is involved in photosynthesis. The Mn/Ca cluster in PSII is responsible for oxygen evolution. Several model systems for this active center have been prepared, mostly polynuclear manganese clusters. However, only a few examples of mixed-metal manganese–calcium clusters similar to the active site have been synthesized.
Constantinos J. Milios, The University of Crete, Herakleion, Greece, and colleagues have synthesized the first two examples of Mn/Ca clusters with amino-acid ligands. The team prepared {[MnIII3CaO(OH)(mAla)(O2CPh)3(HL)2(MeOH)]2} · 6.2 MeCN · 0.9 H2O (1) and [MnIII6CaO3L’6(H2O)3](ClO4)2 · 14.1 H2O (2), with mAla = methylalanine, H3L = 2-(β-naphthalideneamino)-2-hydroxyethyl-1-propanol, and H2L’ = a Schiff-base ligand created from mAlaH and salicylaldehyde. These complexes can be considered possible models for the heterometallic Mn/Ca cluster and its bridging amino acids in PSII.
Based on crystallographic results, the team found that complex 1 contains an (Mn3Ca)2-type cluster, while 2 has a trigonal-prismatic Mn6 cluster with a central Ca. Both complexes show some structural similarities with the active site of PSII. According to the team, other heterometallic Mn/Ca species with natural amino acids as ligands might mimic the site more closely and could be the subject of further studies.
- The first amino acid bound manganese-calcium clusters: a {[MnIII3Ca]2} methylalanine complex, and a [MnIII6Ca] trigonal prism,
Thomais G. Tziotzi, Evangelos K. Andreou, Eirini Tzanetou, Dimitris A Kalofolias, Daniel J. Cutler, Marek Damian Weselski, Milosz Siczek, Tadeusz Lis, Euan K Brechin, Constantinos J Milios,
Dalton Trans. 2020.
https://doi.org/10.1039/d0dt01916j