Compounds with “switchable” properties that can be changed by an external stimulus could have a variety of applications, e.g., in materials science. One example of a switchable property is solvatochromism, a phenomenon in which a solute has different colors in different solvents. Stimulus-responsive materials can, for example, be realized by using weak bonds that can easily be broken and rearranged.
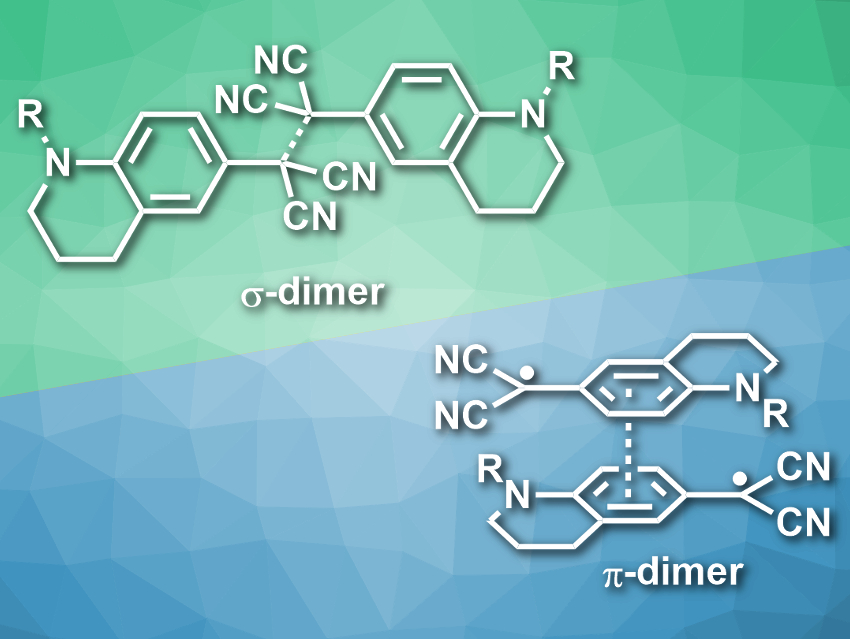
Joshua P. Peterson and Arthur H. Winter, Iowa State University, Ames, USA, have used this weak-bond approach to make solvent-responsive radical dimers that show solvatochromism. The team prepared stable aryl dicyanomethyl radicals (pictured, R = Me, Bu) from aryl malononitrile precursors by oxidation. In toluene at low temperatures, these radicals form colorless σ-dimers. In chloroform at low temperatures, they form π-dimers (pimers), and the solution has a purple color. When the solutions are warmed to room temperature, both types of dimers dissociate, and the existence of free radicals can be observed using electron paramagnetic resonance (EPR) spectroscopy.
The team attributes the change in dimer preference to the polarity of the solvents. The pimer has a higher polarizability and can be stabilized in polar solvents such as chloroform. The less polarizable σ-dimer is formed preferentially in the non-polar toluene. According to the researchers, this type of radical could have applications, e.g., in sensing or in stimuli-responsive polymers.
- Solvent-Responsive Radical Dimers,
Joshua P. Peterson, Arthur H. Winter,
Org. Lett. 2020.
https://doi.org/10.1021/acs.orglett.0c02152