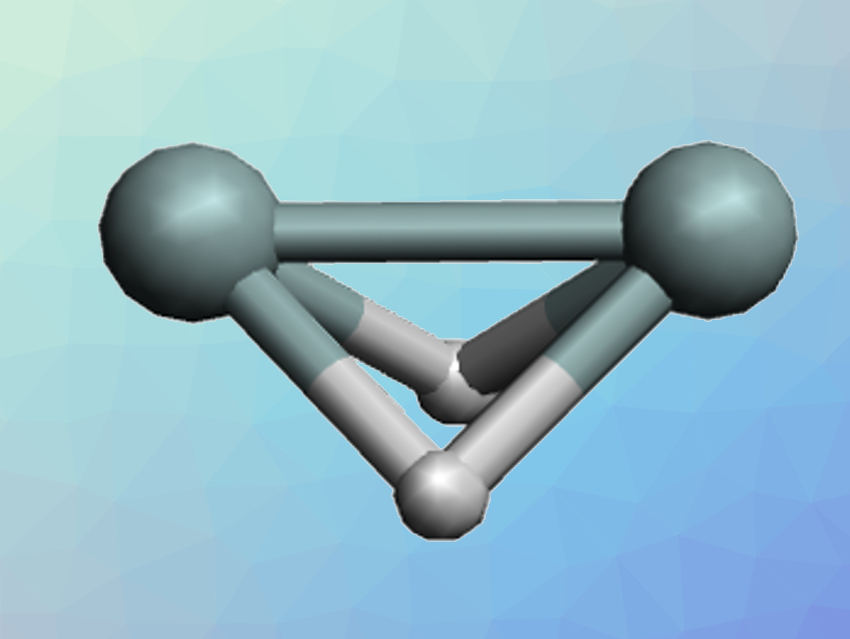
Acetylene or ethyne (C2H2) is a linear molecule with a triple bond. As is often the case, heavier analogs such as Si2H2 have very different structures and a tendency to form single bonds instead of multiple bonds. For Si2H2, the structure with the lowest energy is a dibridged arrangement (pictured). The trans-bent structure (pictured below) is a local minimum on the potential energy surface, i.e., a metastable structure, while the linear H–Si–Si–H arrangement is not a local minimum, i.e., an unstable structure. Quantum chemical calculations can help to understand the bonding in such structures and why they differ from their carbon analogs.
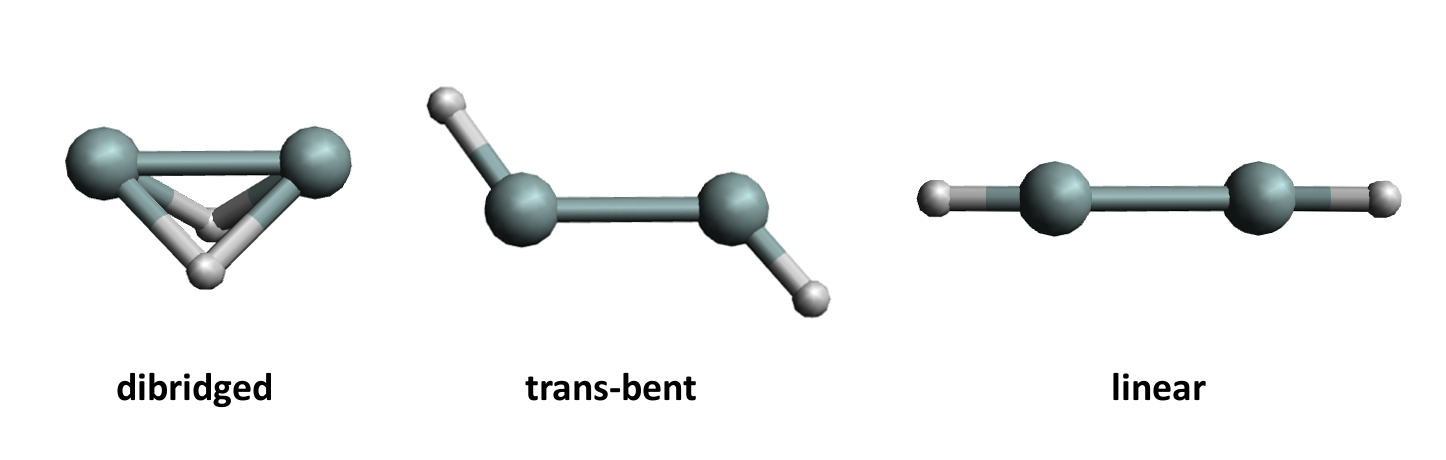
Emilie B. Guidez, University of Colorado, Denver, USA, and colleagues have investigated the relative stabilities of the dibridged, trans-bent, and linear structures of Si2H2 and C2H2 using computational methods. The team calculated both intra- and inter-atomic energy contributions to the bonding energy in the molecules. For this, they used quasi-atomic orbitals (QUAO) based on the actual molecular wave function, without introducing additional assumptions. The intra-atomic energy contributions are generally antibonding and stem from the required electronic modifications of the free atoms in order to form the quasi-atoms in a molecule. The inter-atomic bonding interactions are due to electron sharing between these quasi-atoms.
The stability of the overall structures decreases in the order dibridged < trans-bent < linear for Si2H2. The opposite is true for C2H2, where the linear structure is the most stable by far. Interestingly, the effects of the intra-atomic contributions to the molecular energy follow the same order for both C2H2 and Si2H2: The energy required to form the quasi-atoms increases in the order dibridged < trans-bent < linear. The trend for the inter-atomic bonding contributions is also the same for both C2H2 and Si2H2: the energy released by forming the bonds increases in the order dibridged < trans-bent < linear. However, the absolute values of these energy contributions differ between the elements.
The preferred structures of the silicon- and carbon compound then result from the interplay between these opposing energetic effects. For Si2H2, the unfavorable intra-atomic energy changes outweigh the favorable bonding energy when moving from the dibridged to the linear structure. For C2H2, the opposite is true and the linear structure is preferred.
- Why is Si2H2 Not Linear? An Intrinsic Quasi-Atomic Bonding Analysis,
Emilie B. Guidez, Mark S. Gordon, Klaus Ruedenberg,
J. Am. Chem. Soc. 2020.
https://doi.org/10.1021/jacs.0c03082