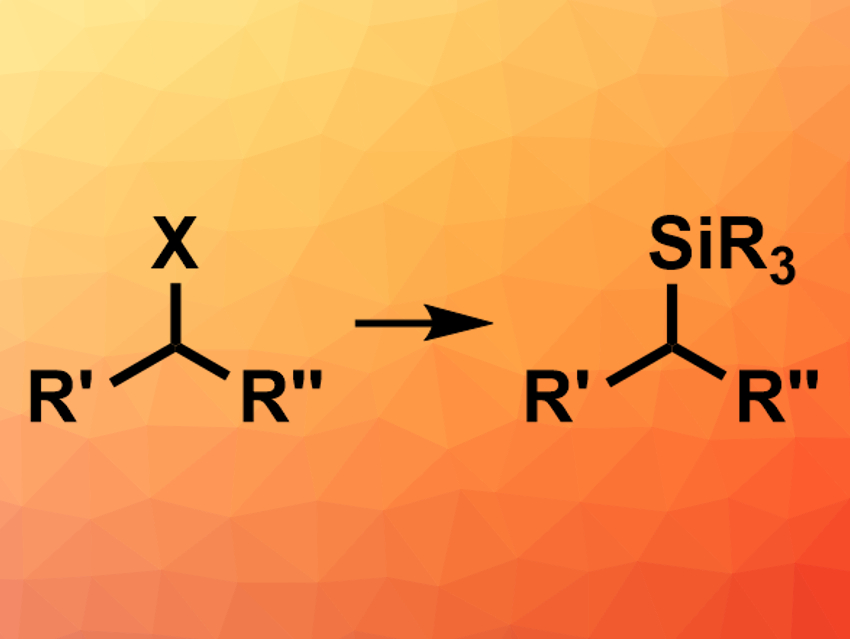
Organosilicon compounds are widely used in organic synthesis. They can be prepared, for example, using hydrosilylation reactions, transition-metal-catalyzed couplings of carbon nucleophiles with silicon electrophiles, or transition-metal-catalyzed couplings of alkyl electrophiles with a silicon nucleophiles. All of these strategies have some limitations, e.g., when it comes to regioselectivity, stereoselectivity, or substrate scope. Thus, new methods for the synthesis of organosilicon compounds are still useful. Couplings of unactivated alkyl electrophiles with silicon nucleophiles to give chiral products, for example, are not well explored.
Armido Studer and colleagues, University of Münster, Germany, have developed a transition-metal-free reaction of unactivated primary/secondary alkyl chlorides or primary alkyl triflates with silyl lithium reagents to give tetraorganosilanes (pictured). The team prepared the silyl lithium reagents from the corresponding commercially available chlorosilanes and elemental lithium. These reagents were then used to convert a variety of alkyl chlorides and primary alkyl triflates into the desired tetraorganosilanes in tetrahydrofuran (THF).
The desired products were obtained in moderate to good yields. The reaction was also used on enantioenriched alkyl chlorides, where it proceeds under inversion of the configuration via an SN2-type mechanism. This provides access to highly enantioenriched chiral alkyl silanes.
- Synthesis of Alkyl Silanes via Reaction of Unactivated Alkyl Chlorides and Triflates with Silyl Lithium Reagents,
Shubhadip Mallick, Ernst-Ulrich Würthwein, Armido Studer,
Org. Lett. 2020.
https://doi.org/10.1021/acs.orglett.0c02337