Cross-coupling reactions are very commonly used in organic synthesis. Often, electrophiles such as aryl bromides, -iodides, or -triflates are used in this type of reaction. Aryl fluorides, in contrast, are much more challenging substrates due to the strength of the C–F bond. Nickel catalysts can help to overcome this limitation. However, existing examples of this approach have fairly limited substrate scopes and do not work well with branched alkylating reagents.
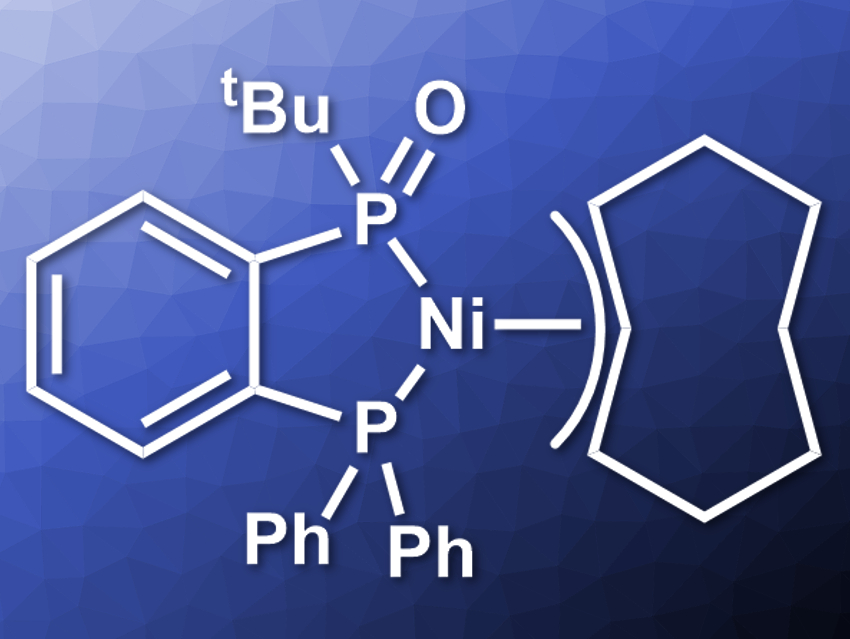
Lutz Ackermann, University of Göttingen, Germany, and colleagues have developed a general strategy for the nickel-catalyzed C–F alkylation of unactivated aryl fluorides with primary and secondary (branched) Grignard reagents. The team used a nickel catalyst with a secondary phosphine oxide (SPO) ligand (pictured), which was prepared from the corresponding bidentate SPO ligand and Ni(cod)2 (cod = 1,5-cyclooctadiene) in toluene at 25 °C.
The catalyst was then used to couple a variety of aryl fluorides and heteroaryl fluorides with primary or secondary alkyl nucleophiles, i.e., Grignard reagents. The reaction was performed under mild conditions at 25–60 °C in tetrahydrofuran (THF). The desired coupling products were obtained in good yields and with high selectvity for the branched products when secondary alkyl nucleophiles were used. Both electron-rich and electron-deficient arenes were coupled successfully.
- C–F Activation for C(sp2)–C(sp3) Cross-Coupling by a Secondary Phosphine Oxide (SPO)-Nickel Complex,
Valentin Müller, Debasish Ghorai, Lorena Capdevila, Antonis M. Messinis, Xavi Ribas, Lutz Ackermann,
Org. Lett. 2020.
https://doi.org/10.1021/acs.orglett.0c02609