Activating white phosphorus (P4) to obtain other phosphorus species, such as phosphines, can be challenging and usually requires the use of hazardous chlorine. Some transition metals can activate P4 in chlorine-free reactions to give phosphorus-containing metal complexes. Several main-group compounds can also react with P4, however, the activation of white phosphorus with aluminium(I) species is not well explored.
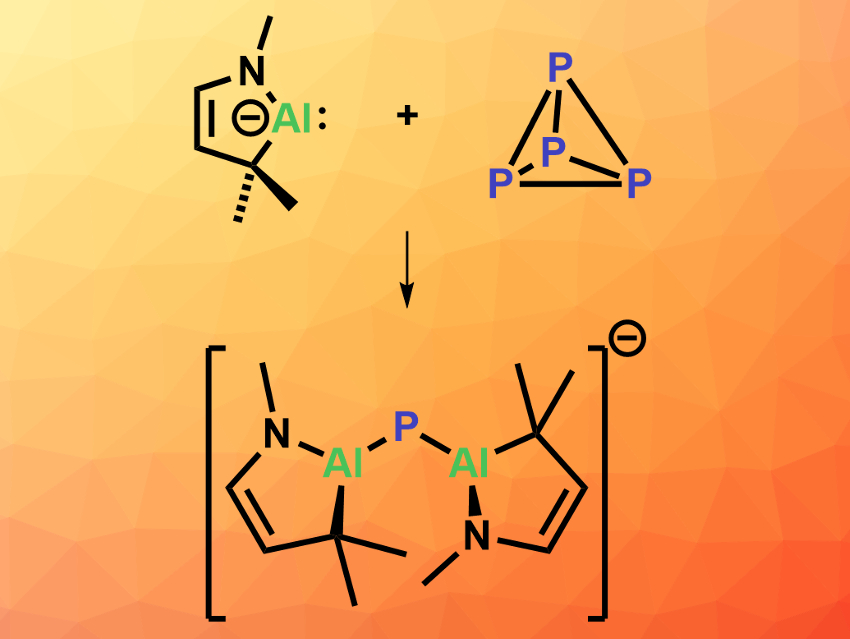
Kota Koshino and Rei Kinjo, Nanyang Technological University, Singapore, have reacted a cyclic (alkyl)(amino)alumanyl anion (CAAAl, simplified structure pictured top left) with P4. The CAAAl was prepared from an α,β-unsaturated imine and AlBr3 in three steps. Adding P4 to the CAAAl species in diethyl ether at room temperature gave a bis(alumanyl)phosphide (pictured above) in a yield of 36 % based on NMR data after 30 min.
To obtain crystals of the product that are suitable for X-ray crystallography, the team started from the dialane dimer that corresponds to the CAAAl, which was reduced using potassium graphite (KC8) and then treated with P4 and 12-crown-4 ether to give the desired bis(alumanyl)phosphide in 27 % isolated yield. The product has a bent structure with an Al–P–Al bond angle of ca. 107° and relatively short Al–P bonds. The work shows that white phosphorus can be fragmented into a P1 unit via activation by a CAAl species, achieving the direct conversion of an anionic Al species to an anionic P species.
- Fragmentation of White Phosphorus by a Cyclic (Alkyl)(Amino)Alumanyl Anion,
Kota Koshino, Rei Kinjo,
Organometallics 2020.
https://doi.org/10.1021/acs.organomet.0c00444