Condensed polycyclic aromatic compounds can have applications in, e.g., organic electronics, optical devices, or magnetic materials. Phenothiazine and phenoxazine, for example, are tricyclic systems with butterfly-like structures. They can be converted into species with planar structures via one-electron oxidations that give the corresponding radical cations. This transformation changes the compounds’ electronic and photophysical properties. Building condensed polycyclic systems that contain phenothiazine or phenoxazine units could, thus, lead to redox-responsive materials.
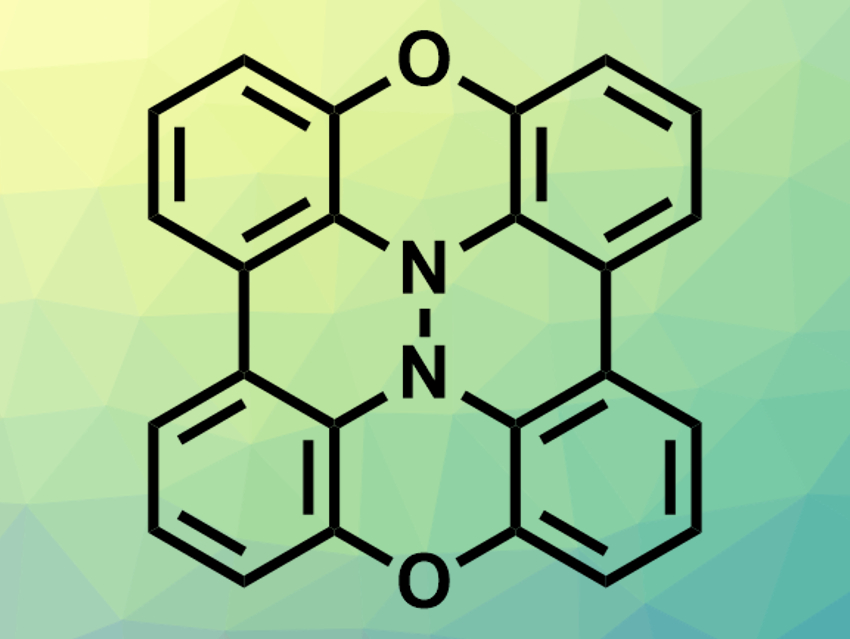
Shuichi Suzuki, Osaka University, Japan, Masatoshi Kozaki, Osaka City University, Japan, and colleagues have synthesized a condensed phenoxazine dimer (BPXZ, pictured) and its radical cation (BPXZ•+). The team prepared BPXZ starting with a Buchwald–Hartwig cross-coupling reaction between 3-bromo-2-iodoanisole and 2-bromo-6-fluoroaniline to give a diarylamine intermediate. The methoxy group was then deprotected using BBr3 to give the corresponding alcohol, followed by an intramolecular aromatic nucleophilic substitution to give 1,9-dibromo-10H-phenoxazine. This phenoxazine intermediate was dimerized via a nickel-mediated homocoupling reaction, and the central N–N bond was formed via aerobic oxidation under alkaline conditions to give BPXZ.
X-ray crystallographic analysis showed that the dimer has a double-butterfly structure, with nitrogen atoms above and below the molecular plane. Analogous to the monomer, this structure could be converted to a planar structure upon oxidation to the radical cation. The oxidation of BPXZ was performed using tris(4-bromophenyl)aminium hexafluoroantimonate as the oxidant in dichloromethane at room temperature. The team obtained the radical cation salt BPXZ•+·SbF6– as a blue solid. Single-crystal X-ray crystallography showed that BPXZ•+ has the expected planar structure. According to the researchers, the condensed dimer might be useful as a building block for redox-responsive magnetic materials.
- Condensed Phenoxazine Dimer and Its Radical Cation,
Takayuki Miyamae, Makoto Haraguchi, Yoshimitsu Tachi, Shuichi Suzuki, Masatoshi Kozaki, Keiji Okada,
Org. Lett. 2020.
https://doi.org/10.1021/acs.orglett.0c02305