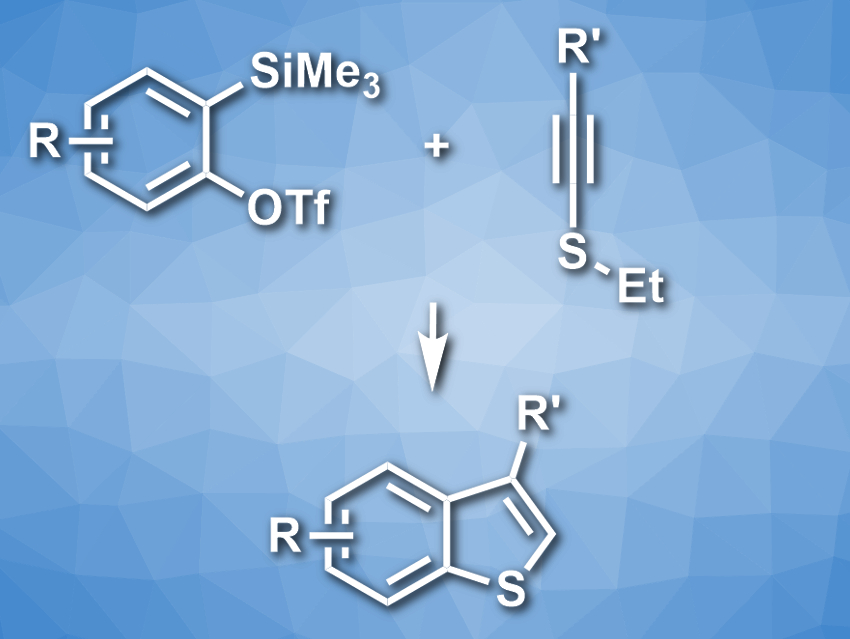
Benzo[b]thiophenes are sulfur-containing heterocyclic structures that are useful, e.g., in pharmaceutical and materials chemistry. However, the preparation of substituted benzothiophenes is still challenging. The existing methods have limited scopes when it comes to the type and location of the introduced substituents.
Suguru Yoshida and colleagues, Tokyo Medical and Dental University (TMDU), Japan, have developed an approach for the synthesis of substituted benzo[b]thiophenes from aryne precursors and alkynyl sulfides (pictured). The team used o-silylaryl triflates as aryne precursors and reacted them with different alkynyl sulfides in the presence of CsF, using acetonitrile as the solvent. The o-silylaryl triflates first form an aryne in situ. The nucleophilic addition of the sulfur atom to this reactive intermediate and a cyclization then give the bicyclic skeleton. A protonation and a deethylation lead to the final product.
The team obtained the desired benzo[b]thiophenes in moderate to good yields. The approach has a good functional group tolerance. It can be used to prepare complex benzothiophene derivatives that are otherwise difficult to access.
- One-step synthesis of benzo[b]thiophenes by aryne reaction with alkynyl sulfides,
Tsubasa Matsuzawa, Takamitsu Hosoya, Suguru Yoshida,
Chem. Sci. 2020.
https://doi.org/10.1039/d0sc04450d