Long molecules can be entangled and tied into knots using, e.g., noncovalent interactions. However, such systems usually allow for only one type of knot.
David A. Leigh, East China Normal University, Shanghai, China, and University of Manchester, UK, and colleagues have synthesized a molecular strand with coordination sites for different metal ions that can be tied into different kinds of knots. The coordination of the metal ions guides the folding of the molecular strand. The same strand can be turned into a three-twist knot, a trefoil knot, or a simple macrocycle by changing the coordinated metal ions.
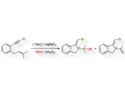
The researchers included two different types of ligands in their strand: three 2,6-pyridine dicarboxamide (pdc) sites and two 1,10-diphenylphenanthroline (dpp) sites. These ligands were connected in the sequence pdc–dpp–pdc–dpp–pdc via short polyethylene oxide chains. Both ends of the strand were functionalized with olefins. The bidentate dpp units can bind to Cu(I) ions and the tridentate pdc groups can bind to lanthanide cations such as Lu(III) ions.
This strand design allowed the team to selectively form different knots. To form a three-twist knot, the team first added Cu(I) ions. This causes the formation of one loop that connects the two dpp groups. Then, a Lu(III) species was added to connect the three pdc groups. The two ends of the strand were joined using ring-closing metathesis. Finally, the metal templates were removed, using potassium cyanide to remove Cu(I) and tetraethylammonium fluoride to remove Lu(III), and the team obtained the desired knot.
Similarly, a trefoil knot can be obtained by adding only Lu(III) and then performing the ring-closing metathesis to connect the chain ends. A simple macrocycle could also be synthesized by ring-closing metathesis in the absence of Cu(I) or Lu(III). According to the researchers, the ability to tie molecular chains into different knots could allow tuning the structure and properties of synthetic oligomers, polymers, and supramolecules.
- Tying different knots in a molecular strand,
David A. Leigh, Fredrik Schaufelberger, Lucian Pirvu, Joakim Halldin Stenlid, David P. August, Julien Segard,
Nature 2020.
https://doi.org/10.1038/s41586-020-2614-0