Transition-metal catalysts are widely used in organic synthesis. Using catalysts based on main-group elements instead could help to reduce costs and environmental impact for many reactions. Germanium catalysis, for example, can be used for the polymerization of lactide or the hydroboration of carbonyls. However, transformations catalyzed by germanium are still rare overall, and extending this limited range would be useful.
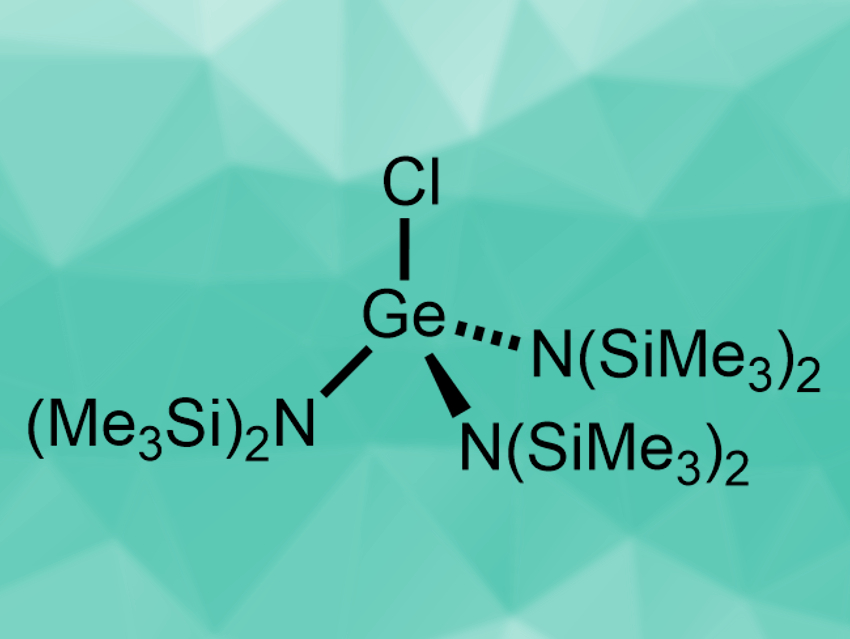
Ruth L. Webster and colleagues, University of Bath, UK, have developed the first hydrophosphination protocol that uses a germanium precatalyst. The precatalyst, [GeCl{N(SiMe3)2}3] (pictured), was prepared by a reaction of GeCl4 with NaN(SiMe3)2. It was then used in reactions of diphenylphosphine with styrenes or internal alkynes at room temperature. The desired hydrophosphination products were obtained in good to excellent yields with anti-Markovnikov selectivity.
The team proposes a reaction mechanism that involves the formation of a germanium-(tris)phosphido complex of the type GeCl(PPh2)3 as the active catalyst. The alkene then inserts into Ge–P bonds and forms alkyl intermediates, and protonolysis gives the final product. According to the researchers, this work could be a basis for the development of other germanium-catalyzed hydrofunctionalization reactions.
- Hydrophosphination using [GeCl{N(SiMe3)2}3] as a pre-catalyst,
Adam N Barrett, Hugh J Sanderson, Mary F Mahon, Ruth L Webster,
Chem. Commun. 2020.
https://doi.org/10.1039/d0cc05792d