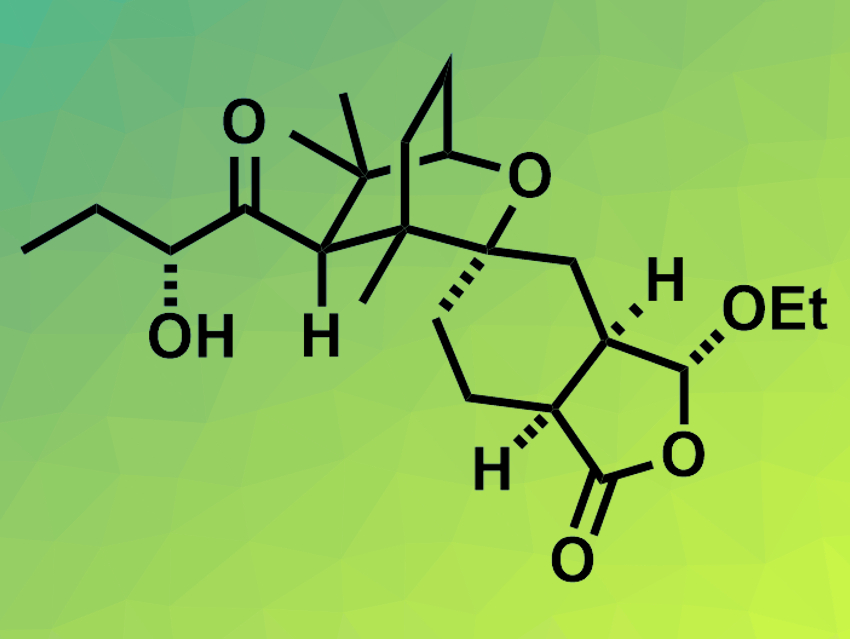
Leonuketal (pictured) is a tetracyclic terpenoid natural product with a complex structure. It was first isolated from Chinese liverwort and belongs to the seco-labdanes, a group of structurally complex and often biologically active natural products. Leonuketal has been shown to be a vasorelaxant, i.e., it can counteract the contraction of blood vessels.
Phillip S. Grant, Daniel P. Furkert, and Margaret A. Brimble, University of Auckland and Maurice Wilkins Centre for Molecular Biodiscovery, both Auckland, New Zealand, have performed the first total synthesis of leonuketal. Key steps in the synthesis are a Ti(III)-mediated reductive cyclization of an epoxy nitrile ether to give a bicyclic ketone intermediate, followed by an unusual ring-opening alkyne formation, and an unprecedented Au(I)-catalyzed cyclization of a β-keto(enol)lactone to assemble the core spiroketal motif.
Overall, the synthesis has 23 steps starting from the epoxy nitrile ether. (±)-Leonuketal was obtained in a diastereomeric ratio of 1:1. According to the researchers, the work could have applications in the preparation of other complex structures and allow further investigations of the chemical and biological properties of leonuketal and similar natural products.
- Total Synthesis of (±)-Leonuketal,
Phillip S. Grant, Daniel P. Furkert, Margaret A. Brimble,
Org. Lett. 2020.
https://doi.org/10.1021/acs.orglett.0c03364



![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)