Carboranes are molecular clusters composed of boron, carbon, and hydrogen atoms, held together by delocalized bonds. They have applications, e.g., in supramolecular and pharmaceutical chemistry. Icosahedral carboranes with twelve vertices have been extensively studied, while the chemistry of so-called supercarboranes with more than twelve vertices is less well developed. Several 13- and 14-vertex closo-carboranes have been prepared, as well as a 15-vertex metallacarborane with a ruthenium atom. Metal-free carboranes with more than 14 vertices, in contrast, had remained elusive so far.
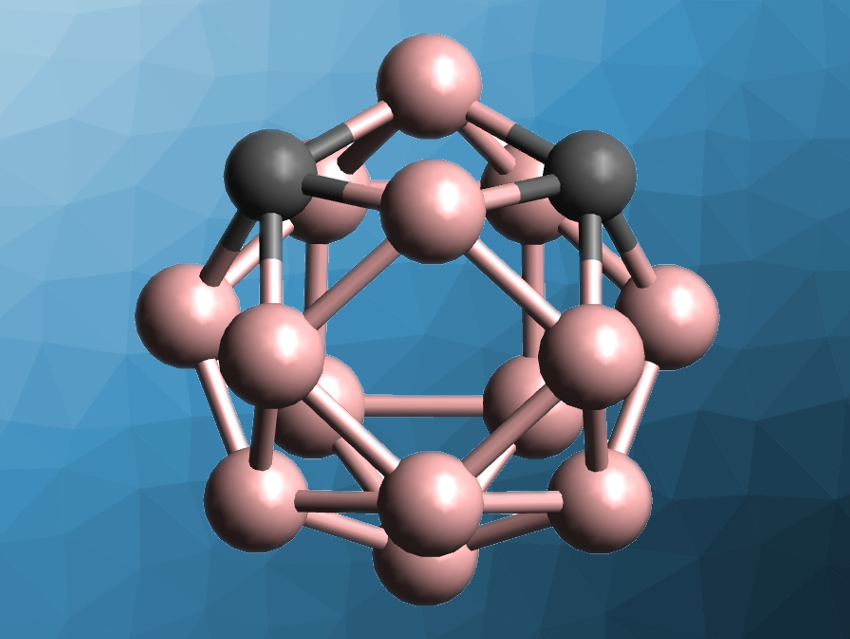
Zuowei Xie, The Chinese University of Hong Kong, Shatin, Hong Kong, China, and colleagues have synthesized 15- and 16-vertex closo-carboranes as well as a 16-vertex ruthenacarborane. The team used silyl groups at the carbon vertices to stabilize the starting materials, 1,2-(RMe2Si)2-1,2-C2B10H10 (R = Me, Ph). These compounds were first treated with sodium metal in tetrahydrofuran (THF) at room temperature and then with HBBr2·SMe2 in dimethoxyethane (DME) at −78–25 °C to give 13-vertex closo-carboranes. Additional rounds of the same reagents led to another cage expansion each time, giving 14-, 15-, and 16-vertex closo-carboranes (example pictured, structure simplified).
When the team reacted the 15-vertex closo-carborane with sodium followed by [(p-cymene)RuCl2]2, they obtained a 16-vertex ruthenacarborane. According to the researchers, a similar strategy with silyl groups at the cage boron atoms could allow the synthesis of carboranes with 17 vertices or more. Such superclusters could have applications, e.g., in coordination chemistry and materials science.
- Synthesis and X-ray characterization of 15- and 16-vertex closo-carboranes,
Fangrui Zheng, Tsz Hin Yui, Jiji Zhang, Zuowei Xie,
Nat. Commun. 2020.
https://doi.org/10.1038/s41467-020-19661-5