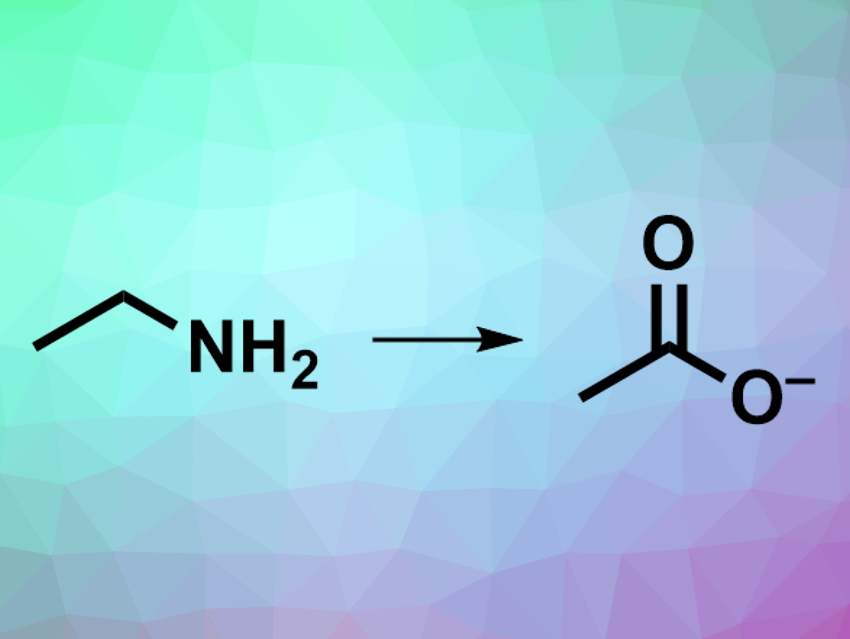
The synthesis of amines via the amination of alcohols or ketones is common in organic chemistry. The reverse reaction, i.e., the deamination of primary amines, is more challenging. Enzymatic oxidative deaminations of primary amines in living organisms are well-known processes. The use of these enzymes in organic synthesis, however, is hindered by a narrow substrate scope, and other synthetic methods for oxidative deamination usually require harsh oxidants or sacrificial reagents.
David Milstein, Weizmann Institute of Science, Rehovot, Israel, and colleagues have developed an efficient, green process for oxidative deamination that uses water as the oxidant and releases hydrogen gas. The team used an acridine-based ruthenium pincer complex as the catalyst to convert a variety of primary amines to the corresponding carboxylates (pictured) in an alkaline water/dioxane solution at 150 °C. Branched aliphatic amines were converted to ketones.
Depending on the substrates, the desired products were obtained in moderate to excellent yields. The method does not require a sacrificial oxidant that generates waste. The approach can also be used for the oxidative deamination of amides to produce carboxylates.
- Catalytic Oxidative Deamination by Water with H2 Liberation,
Shan Tang, Michael Rauch, Michael Montag, Yael Diskin-Posner, Yehoshoa Ben-David, David Milstein,
J. Am. Chem. Soc. 2020.
https://doi.org/10.1021/jacs.0c10826