Polyketides from marine sources can have applications, e.g., as anti-cancer drugs. However, they are only available in small amounts from natural sources and are mostly non-crystalline. This makes the determination of their exact structure and stereochemistry challenging.
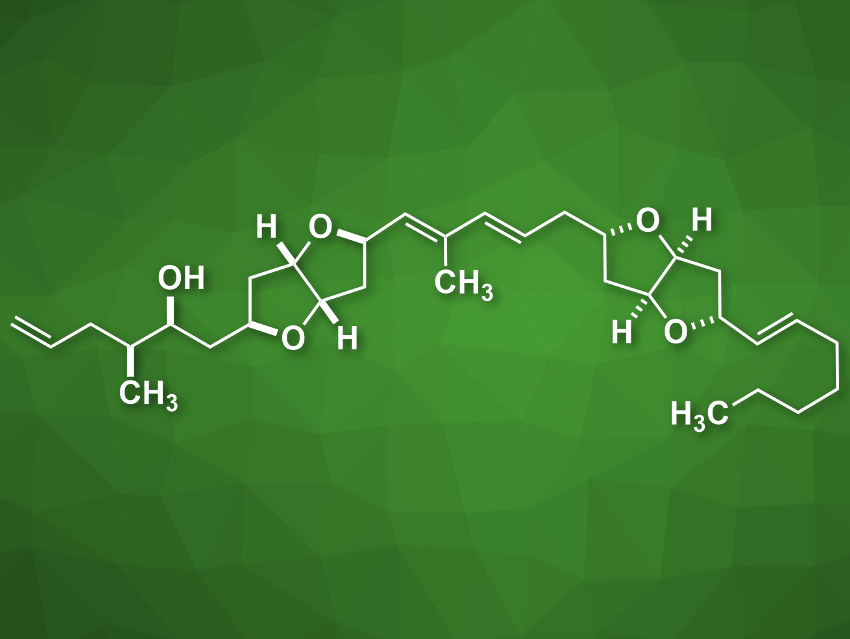
Amphirionin-2 (pictured) is a linear polyketide metabolite that was isolated from marine dinoflagellate cells. It shows cytotoxic activity against different human cancer cell lines. Its putative structure had been assigned using 2D NMR spectroscopy, but its complete stereochemical assignment had not been achieved so far.
Haruhiko Fuwa, Chuo University, Tokyo, Japan, and colleagues have performed the first total synthesis of amphirionin-2 and determined its absolute configuration. The team synthesized four candidate stereoisomers via a unified synthesis process in order to compare their properties with natural amphirionin-2. They used cobalt-catalyzed Mukaiyama-type cyclizations of γ-hydroxy olefins for the stereocontrolled construction of the tetrahydrofuran rings of the target compounds. A late-stage Stille-type reaction allowed the team to combine two hexahydrofuro[3,2-b]furan moieties in a convergent manner and provided easy access to the four target stereoisomers.
Overall, the products were obtained in 19 linear steps from benzyloxyacetaldehyde. Based on 1H and 13C NMR spectroscopy and chiral HPLC chromatography data, the team was able to assign the correct stereochemical structure of amphirionin-2, which involved a reassignment of the C5/C7 relative configuration compared with previously proposed structures.
- Total synthesis and complete configurational assignment of amphirionin-2,
Shota Kato, Daichi Mizukami, Tomoya Sugai, Masashi Tsuda, Haruhiko Fuwa,
Chem. Sci. 2021.
https://doi.org/10.1039/d0sc06021f