Cascade reactions are efficient processes for forming several new chemical bonds in a single step. They can, e.g., be based on radical or cationic intermediates. Radical cascade reactions often have drawbacks such as a need for toxic or hazardous reagents. Visible-light photocatalysis can promote single-electron transfers, and thus, act as an alternative method to initiate radical cascades. Palladium-phosphine complexes, which combine the properties of photocatalysts and cross-coupling-catalysts, are particularly useful for this type of reaction.
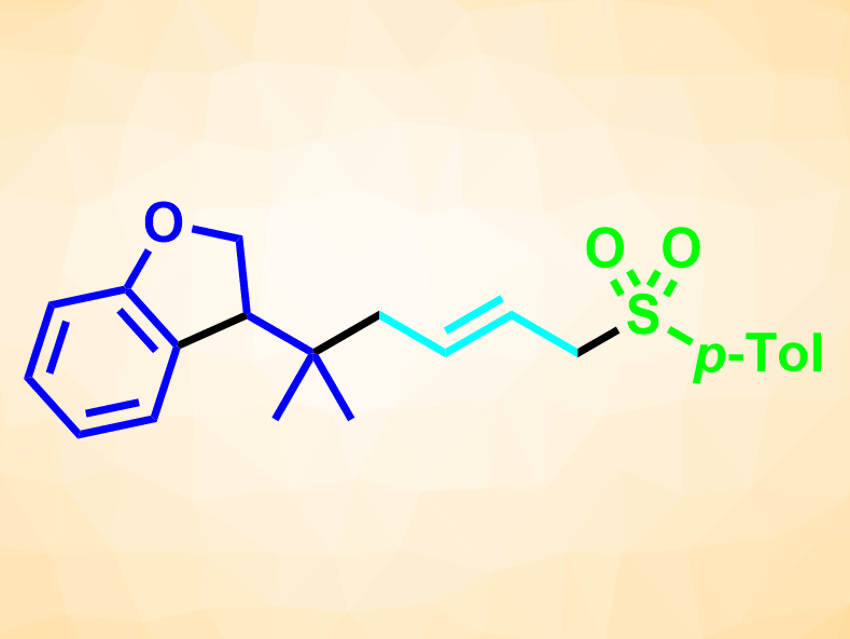
Frank Glorius and colleagues, University of Münster, Germany, have developed a three-component radical cascade reaction that involves a radical cyclization/allylic substitution sequence between alkyl/aryl bromides, 1,3-dienes, and nucleophiles. This type of reaction forms three new bonds in one step (example product pictured, newly formed bonds pictured in black). The team used Pd(PPh3)4 as a catalyst together with bis[(2-diphenylphosphino)phenyl] ether (DPEPhos) as a ligand, K2CO3 as a base, and 1,4-dioxane as the solvent. The reactions were performed under blue LED light at 25–30 °C.
The team performed over 80 different examples of this cascade reaction, using both (hetero-)aryl and alkyl bromides, different substituted dienes, and a variety of nitrogen, sulfur, carbon, and oxygen nucleophiles, e.g., amines, sulfinates, 1,3-dicarbonyls, and phenols. The desired products were obtained in moderate to excellent yields. The team also used the reaction in a flow process, which represents the first time a palladium-catalyzed photoredox reaction involving radical species was performed using flow chemistry.
- Three-Component Three-Bond Forming Cascade via Palladium Photoredox Catalysis,
Peter Bellotti, Maximilian Koy, Christian Gutheil, Steffen Heuvel, Frank Glorius,
Chem. Sci. 2020.
https://doi.org/10.1039/d0sc05551d