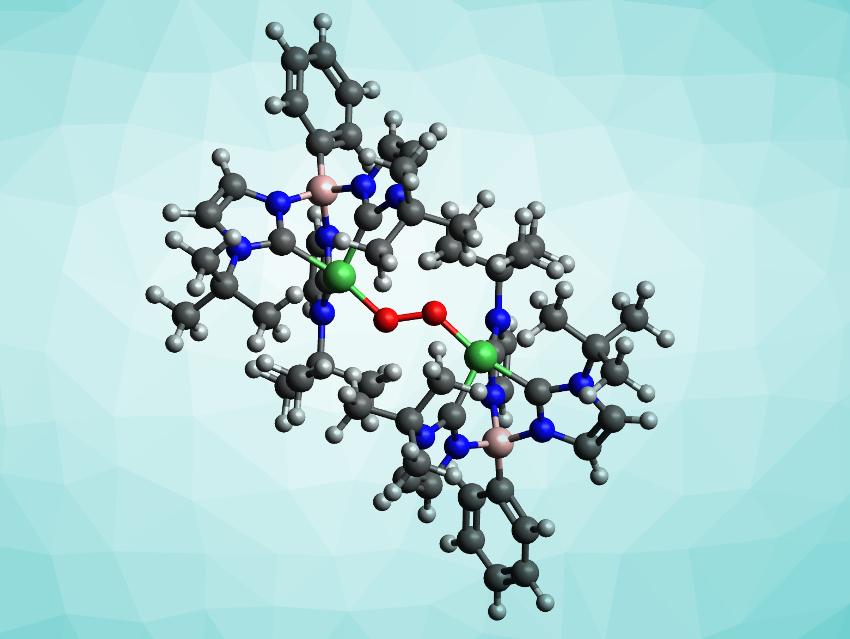
High-valent oxo-, peroxo-, and superoxo transition-metal complexes are important in both biological and synthetic systems, e.g., for the oxidation of organic substrates or water oxidation. However, their high reactivity can make the isolation of such complexes challenging. There are several examples of high-valent oxygenated complexes of Mn, Fe, Co, and Cu, but oxygenated Ni complexes are much rarer. For example, no peroxo-bridged high-valent Ni complexes had been known so far.
John S. Anderson, University of Chicago, IL, USA, and colleagues have synthesized and isolated the first example of a NiIII2(μ-1,2-peroxo) complex, {[PhB(tBuIm)3]Ni–O–O–Ni[(tBuIm)3BPh]}{BArF4}2 (cation pictured, BArF4 = tetrakis(3,5-bis(trifluoromethyl)phenyl)borate). These complexes have bulky outer ligands with three carbene units (tBuIm) each, connected via a boron atom. These ligands each coordinate a Ni(III) ion, and the two resulting units are connected via a peroxo bridge.
The complex was synthesized from the chloride precursor [PhB(tBuIm)3]NiCl. The precursor was treated with NaBArF4 in dichloromethane (DCM) to form a dimeric intermediate. A reaction with dry O2 at −78 °C then gave the desired peroxo complex. Single-crystal X-ray diffraction confirmed its structure. The complex is stable toward oxygen dissociation, but reactive towards both nucleophiles and electrophiles.
- Generation and Reactivity of a NiIII2(μ-1,2-peroxo) Complex,
Norman Zhao, Alexander S. Filatov, Jiaze Xie, Ethan A. Hill, Andrey Yu. Rogachev, John S. Anderson,
J. Am. Chem. Soc. 2020.
https://doi.org/10.1021/jacs.0c10958