Catechol, or 1,2-dihydroxybenzene, is commonly used as a precursor in organic synthesis. Catechol derivatives are often used as nucleophiles, but are also redox-active. Under air, they can undergo one- or two-electron oxidations to semiquinone radicals or ortho-quinones.
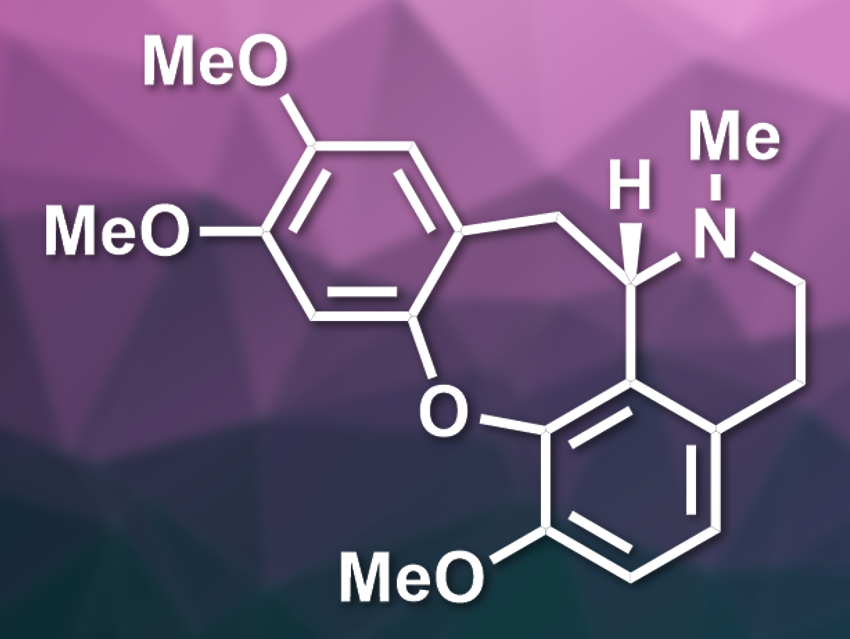
Jean-Philip Lumb, McGill University, Montreal, Canada, and colleagues have used the autoxidation of a catechol for the formation of the central seven-membered ring in the total synthesis of the natural product (S)-cularine (pictured). For this, the team used a metal-free cyclization of a substituted catechol precursor in the presence of air and tetrabutylammonium fluoride (TBAF). Formally, this reaction is a nucleophilic aromatic substitution on the catechol, forming a new C–O bond.
According to the researchers, this challenging C–O bond formation is promoted by the formation of an intermediate ortho-quinone via autoxidation of the catechol precursor in air. This type of transformation could expand the scope of reactions involving catechols.
- Total Synthesis of (S)-Cularine via Nucleophilic Substitution on a Catechol,
Zheng Huang, Xiang Ji, Jean-Philip Lumb,
Org. Lett. 2020.
https://doi.org/10.1021/acs.orglett.0c04000