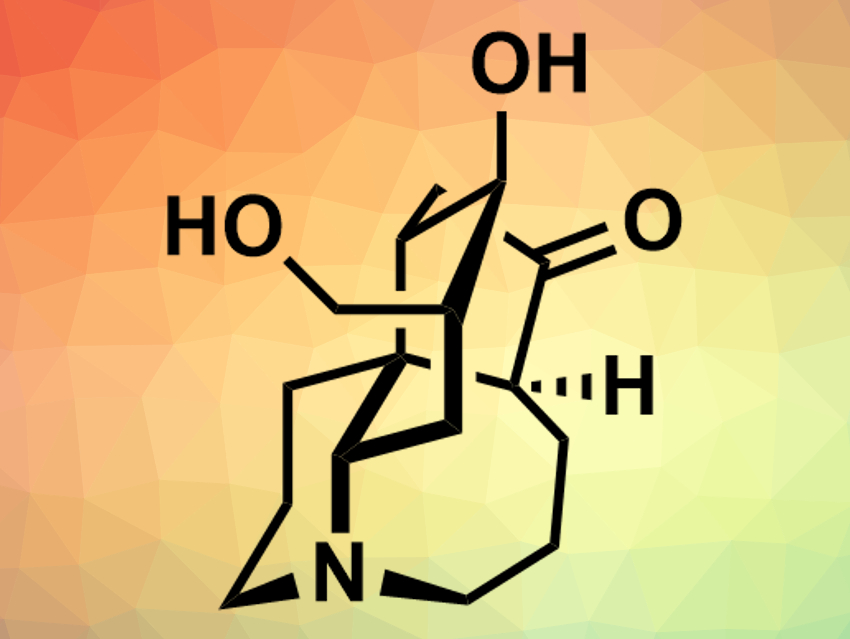
The natural products lyconesidine A and lyconesidine B (pictured) were first isolated from the club moss Lycopodium chinense. These alkaloids, which differ only in one hydroxy group, have a complex, tetracyclic structure with a central quarternary carbon atom. Both compounds have a cytotoxic effect against certain mouse cancer cell lines, as well as other bioactivities. So far, there had been no reported total syntheses of lyconesidines.
Chihiro Tsukano, Yoshiji Takemoto, Kyoto University, Japan, and colleagues have performed a total synthesis of lyconesidine B. The team started from δ-valerolactam, which was converted to an enol triflate and subjected to a Suzuki–Miyaura coupling with a vinyl boronic ester. After several further functionalizations, including the introductions of an aldehyde group and a diazonitrile unit, a cascade reaction was used to form a decahydroquinoline system. Further transformations led to a precursor for a domino ene-yne cyclization, which was then used to close the remaining two rings. Finally, the remaining functional groups were installed on the compound’s skeleton to give lyconesidine B.
Lyconesidine B was synthesized in 30 steps from the enol triflate with an overall yield of 0.52 %. The mass and NMR spectra of the compound were in agreement with the natural product. According to the researchers, the overall synthetic strategy could also be applied to analogues of lyconesidine B, whose biological activities could then be studied.
- Total Synthesis of Lyconesidine B, a Lycopodium Alkaloid with an Oxygenated, Amine-Type Fawcettimine Core,
Tomohiro Kurose, Chihiro Tsukano, Takeshi Nanjo, Yoshiji Takemoto,
Org. Lett. 2020.
https://doi.org/10.1021/acs.orglett.0c03816