Several compounds containing planar tetracoordinated carbon (ptC), the so-called anti-van’t Hoff/Le Bel carbon, are experimentally known. However, stable systems containing flat tetracoordinated silicon (ptSi) are hardly known. There is one example of a planar silicon in the gas phase, but it has never been isolated.
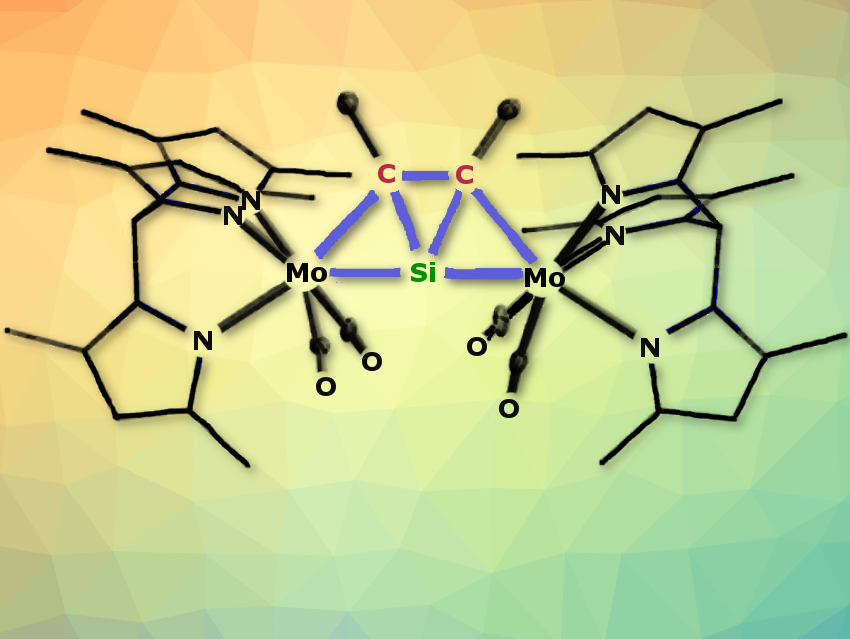
Alexander C. Filippouand colleagues, University of Bonn, Germany, have synthesized a series of thermally stable complexes of the general formula [Tp′(CO)2MSiC(R1)C(R2)M(CO)2Tp′] (M = Mo, W; R1 = R2 = Me or R1 = H, R2 = SiMe3, Ph; Tp′ = κ3-N,N′,N″-hydridotris(3,5-dimethylpyrazolyl)borate). They incorporate a ptSi atom in addition to two ptC atoms (pictured above). To make these flat tetravalent silicon molecules, the team reacted the metallasilylidyne complexes [Tp′(CO)2M≡Si–M(CO)2(PMe3)Tp′] with alkynes R1C≡CR2. The bright yellow compounds are only moderately sensitive to air and decompose only when heated to over 200 °C.
The ptSi atom is located in a tricyclic trapezoidal core with one inner SiC2 and two outer M-Si-C three-membered rings. The rings are fused via two Si–C bonds. The molecule has a nearly linear M–Si–M backbone, short M–Si bonds, long M–C bonds, and two planar tetracoordinated carbon atoms. Anti-van’t Hoff/Le Bel carbon and silicon centers are adjacent. The aromaticity of the central SiC2 ring with two delocalized π-electrons and the two metal centers stabilize the ptSi geometry.
- Planar Tetracoordinated Silicon (ptSi): Room-Temperature Stable Compounds Containing Anti-van’t Hoff/Le Bel Silicon,
Priyabrata Ghana, Jens Rump, Gregor Schnakenburg, Marius I. Arz, Alexander C. Filippou,
J. Am. Chem. Soc. 2020.
https://doi.org/10.1021/jacs.0c11628